Expression of Immunoglobulin G4 in Eosinophilic Esophagitis
- PMID: 38673448
- PMCID: PMC11050440
- DOI: 10.3390/jcm13082175
Expression of Immunoglobulin G4 in Eosinophilic Esophagitis
Abstract
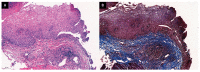
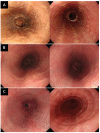
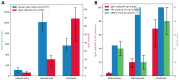
Background: Eosinophilic esophagitis (EoE) is a disease that has been subcategorized into two endoscopic phenotypes: inflammatory and fibrostenotic. Moreover, studies have shown a link between EoE and immunoglobulin G4 (IgG4), a subclass of the immunoglobulin G (IgG) antibody. In this study, we aimed to evaluate the relationship between histologic IgG4 expression and endoscopic phenotypes in patients with EoE. Methods: This case-control study included patients diagnosed with EoE (n = 19) and patients with non-obstructive dysphagia without abnormal findings as controls (NOD; n = 12). The EoE group was further divided into three subgroups based on endoscopic phenotype: inflammatory, fibrostenotic, or combined. Retrospective examination of endoscopic findings and pathological slides was performed to analyze IgG4 staining. Results: Histological analysis revealed a significant difference in IgG4 cell count (15.00 vs. 0.58, p = 0.003) and eosinophil cell count (84.67 vs. 0.08, p < 0.001) between the EoE and NOD groups. Symptom manifestation and blood test results were similar across all three endoscopic EoE phenotypes. However, histological analysis revealed a significant difference in IgG4 cell count between the inflammatory, fibrostenotic, and combined phenotypes (4.13 vs. 17.6 vs. 59.7, p = 0.030). Conclusions: IgG4 expression was higher in EoE patients than in those with NOD, the highest being in the combined phenotype subgroup. These findings emphasize the important role of endoscopic and histological examination in diagnosing EoE and the need for further research in this area.
Keywords: EoE endoscopic phenotype; eosinophilic esophagitis; immunoglobulin G.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Furuta G.T., Liacouras C.A., Collins M.H., Gupta S.K., Justinich C., Putnam P.E., Bonis P., Hassall E., Straumann A., Rothenberg M.E. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–1363. doi: 10.1053/j.gastro.2007.08.017. - DOI - PubMed
-
- Park H. Eosinophilic esophagitis. Korean J. Gastroenterol. 2007;50:286–291. - PubMed
LinkOut - more resources
Full Text Sources

