Examining Lurasidone Efficacy in Patients with Schizophrenia Spectrum Illness and Concurrent Alcohol and Substance Use Disorder: A Prospective, Multicentric, Real-World Investigation
- PMID: 38673478
- PMCID: PMC11051375
- DOI: 10.3390/jcm13082206
Examining Lurasidone Efficacy in Patients with Schizophrenia Spectrum Illness and Concurrent Alcohol and Substance Use Disorder: A Prospective, Multicentric, Real-World Investigation
Abstract
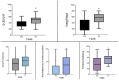
Background: Dual disorders (DD) entail the coexistence of a substance use disorder (SUD) and another mental health condition, often within psychotic and affective disorders. This study aims to evaluate lurasidone, an innovative atypical antipsychotic, in individuals diagnosed with schizophrenia spectrum disorder and concurrent comorbidities of alcohol use disorder/substance use disorder (AUD/SUD). Methods: A cohort of 23 subjects diagnosed with schizophrenia spectrum disorder and comorbid AUD/SUD underwent psychometric assessments at baseline (T0) and one-month (T1) post-lurasidone initiation. Results: Lurasidone exhibited significant reductions in psychopathological burden, evidenced by decreased total PANSS scores (Z = 2.574, p = 0.011). Positive symptoms, substance craving (VAS Craving; Z = 3.202, p = 0.001), and aggressivity (MOAS scale; Z = 2.000, p = 0.050) were notably reduced. Clinical Global Impression (CGI) scores significantly improved (Z = 2.934, p = 0.003). Quality of life enhancements were observed in SF-36 subscales (energy, emotional well-being, and social functioning) (p < 0.05) and Q-LES-Q-SF scale (Z = -2.341, p = 0.021). A safety analysis indicated lurasidone's good tolerability, with only 8.7% reporting discontinuation due to side effects. Conclusions: This study offers initial evidence supporting lurasidone's efficacy and safety in dual diagnoses, highlighting positive effects on psychopathology, substance craving, and quality of life. These findings emphasize the need for tailored, comprehensive treatment strategies in managing the complexities of this patient population.
Keywords: atypical antipsychotic; dual disorder; schizophrenia spectrum disorder; substance use disorder.
Conflict of interest statement
G.M. has been a consultant and/or a speaker and/or has received research grants from Angelini, Doc Generici, Janssen-Cilag, Lundbeck, Otsuka, Pfizer, Servier, and Recordati. All the other authors declare no conflicts of interest.
Figures
Similar articles
-
Investigating the Effectiveness of Brexpiprazole in Subjects with Schizophrenia Spectrum Illness and Co-Occurring Substance Use Disorder: A Prospective, Multicentric, Real-World Study.Pharmaceuticals (Basel). 2024 Apr 21;17(4):535. doi: 10.3390/ph17040535. Pharmaceuticals (Basel). 2024. PMID: 38675495 Free PMC article.
-
A review of published evidence reporting on the efficacy and pharmacology of lurasidone.Expert Opin Pharmacother. 2012 Aug;13(11):1653-9. doi: 10.1517/14656566.2012.683174. Epub 2012 May 5. Expert Opin Pharmacother. 2012. PMID: 22559286 Review.
-
Brexpiprazole in patients with schizophrenia with or without substance use disorder: an observational study.Front Psychiatry. 2023 Dec 4;14:1321233. doi: 10.3389/fpsyt.2023.1321233. eCollection 2023. Front Psychiatry. 2023. PMID: 38111619 Free PMC article.
-
Lurasidone in the treatment of schizophrenia: a 6-week, placebo-controlled study.Psychopharmacology (Berl). 2013 Feb;225(3):519-30. doi: 10.1007/s00213-012-2838-2. Epub 2012 Aug 19. Psychopharmacology (Berl). 2013. PMID: 22903391 Free PMC article. Clinical Trial.
-
Lurasidone in schizophrenia: new information about dosage and place in therapy.Adv Ther. 2012 Oct;29(10):815-25. doi: 10.1007/s12325-012-0052-6. Epub 2012 Sep 20. Adv Ther. 2012. PMID: 23001538 Review.
Cited by
-
Efficacy of Lurasidone in First-Episode Psychosis: Patient Phenotypes, Dosage, and Recommendations from an Expert Panel.Neurol Ther. 2025 Feb;14(1):85-98. doi: 10.1007/s40120-024-00700-y. Epub 2025 Jan 6. Neurol Ther. 2025. PMID: 39760831 Free PMC article. Review.
-
Box-Behnken optimized MPA-CdTe quantum dots as turn-off fluorescent probes for sensitive lurasidone determination in pharmaceutical, biological, and environmental matrices.RSC Adv. 2025 Mar 24;15(12):8855-8866. doi: 10.1039/d5ra00519a. eCollection 2025 Mar 21. RSC Adv. 2025. PMID: 40129648 Free PMC article.
-
A Latent Class Analysis of Psychopathology Among Adults in Residential Substance Use Treatment: Associations With Craving and Treatment Dropout.Subst Use. 2025 May 15;19:29768357251337850. doi: 10.1177/29768357251337850. eCollection 2025 Jan-Dec. Subst Use. 2025. PMID: 40385353 Free PMC article.
-
Over-the-counter Psychosis: A Systematic Review of the Misuse of Antihistamines, Cough Medicines, and Decongestants and the Risk of Developing Psychosis.Curr Neuropharmacol. 2025;23(8):956-973. doi: 10.2174/011570159X344365250114064248. Curr Neuropharmacol. 2025. PMID: 39976040 Free PMC article.
References
-
- Adan A., Benaiges I. Neuropathology of Drug Addictions and Substance Misuse. Elsevier; Amsterdam, The Netherlands: 2016. Comorbidity between Substance Use Disorder and Severe Mental Illness; pp. 258–268. - DOI
-
- Martinotti G., Chiappini S., Mosca A., Miuli A., Santovito M.C., Pettorruso M., Skryabin V., Sensi S.L., Giannantonio M.D. Atypical Antipsychotic Drugs in Dual Disorders: Current Evidence for Clinical Practice. Curr. Pharm. Des. 2022;28:2241–2259. doi: 10.2174/1381612828666220623092853. - DOI - PubMed
-
- EMCDDA . European Drug Report 2023: Trends and Developments. EMCDDA; Lisbon, Portugal: 2023.
LinkOut - more resources
Full Text Sources