Effects of Slow Freezing and Vitrification of Human Semen on Post-Thaw Semen Quality and miRNA Expression
- PMID: 38673743
- PMCID: PMC11050687
- DOI: 10.3390/ijms25084157
Effects of Slow Freezing and Vitrification of Human Semen on Post-Thaw Semen Quality and miRNA Expression
Abstract
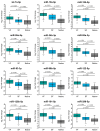
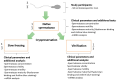
Semen cryopreservation has played an important role in medically assisted reproduction for decades. In addition to preserving male fertility, it is sometimes used for overcoming logistical issues. Despite its proven clinical usability and safety, there is a lack of knowledge of how it affects spermatozoa at the molecular level, especially in terms of non-coding RNAs. Therefore, we conducted this study, where we compared slow freezing and vitrification of good- and poor-quality human semen samples by analyzing conventional sperm quality parameters, performing functional tests and analyzing the expression of miRNAs. The results revealed that cryopreservation of normozoospermic samples does not alter the maturity of spermatozoa (protamine staining, hyaluronan binding), although cryopreservation can increase sperm DNA fragmentation and lower motility. On a molecular level, we revealed that in both types of cryopreservation, miRNAs from spermatozoa are significantly overexpressed compared to those in the native semen of normozoospermic patients, but in oligozoospermic samples, this effect is observed only after vitrification. Moreover, we show that expression of selected miRNAs is mostly overexpressed in native oligozoospermic samples compared to normozoospermic samples. Conversely, when vitrified normozoospermic and oligozoospermic samples were compared, we determined that only miR-99b-5p was significantly overexpressed in oligozoospermic sperm samples, and when comparing slow freezing, only miR-15b-5p and miR-34b-3p were significantly under-expressed in oligozoospermic sperm samples. Therefore, our results imply that cryopreservation of normozoospermic sperm samples can modulate miRNA expression profiles in spermatozoa to become comparable to those in oligozoospermic samples.
Keywords: assisted reproduction; cryopreservation; infertility; microRNA; semen; slow freezing; spermatozoa; vitrification.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Smeenk J., Wyns C., De Geyter C., Bergh C., Cuevas I., de Neubourg D., Kupka M.S., Rezabek K., Rugescu I., Tandler-Schneider A., et al. Assisted Reproductive Technology (ART) in Europe 2020 and development of a strategy of vigilance: Preliminary results generated from European registers by the ESHRE EIM Consortium. Hum. Reprod. 2023;38((Suppl. S1)):dead093.014. doi: 10.1093/humrep/dead093.186. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

