Car T Cells in Solid Tumors: Overcoming Obstacles
- PMID: 38673757
- PMCID: PMC11050550
- DOI: 10.3390/ijms25084170
Car T Cells in Solid Tumors: Overcoming Obstacles
Abstract
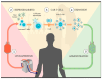
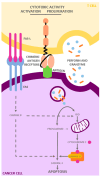
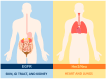
Chimeric antigen receptor T cell (CAR T cell) therapy has emerged as a prominent adoptive cell therapy and a therapeutic approach of great interest in the fight against cancer. This approach has shown notorious efficacy in refractory hematological neoplasm, which has bolstered its exploration in the field of solid cancers. However, successfully managing solid tumors presents considerable intrinsic challenges, which include the necessity of guiding the modified cells toward the tumoral region, assuring their penetration and survival in adverse microenvironments, and addressing the complexity of identifying the specific antigens for each type of cancer. This review focuses on outlining the challenges faced by CAR T cell therapy when used in the treatment of solid tumors, as well as presenting optimizations and emergent approaches directed at improving its efficacy in this particular context. From precise localization to the modulation of the tumoral microenvironment and the adaptation of antigen recognition strategies, diverse pathways will be examined to overcome the current limitations and buttress the therapeutic potential of CAR T cells in the fight against solid tumors.
Keywords: cancer; immunotherapy; solid tumors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

