Cellular Imaging and Time-Domain FLIM Studies of Meso-Tetraphenylporphine Disulfonate as a Photosensitising Agent in 2D and 3D Models
- PMID: 38673807
- PMCID: PMC11050357
- DOI: 10.3390/ijms25084222
Cellular Imaging and Time-Domain FLIM Studies of Meso-Tetraphenylporphine Disulfonate as a Photosensitising Agent in 2D and 3D Models
Abstract
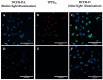
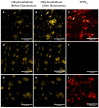
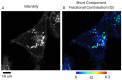
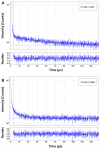
Fluorescence lifetime imaging (FLIM) and confocal fluorescence studies of a porphyrin-based photosensitiser (meso-tetraphenylporphine disulfonate: TPPS2a) were evaluated in 2D monolayer cultures and 3D compressed collagen constructs of a human ovarian cancer cell line (HEY). TPPS2a is known to be an effective model photosensitiser for both Photodynamic Therapy (PDT) and Photochemical Internalisation (PCI). This microspectrofluorimetric study aimed firstly to investigate the uptake and subcellular localisation of TPPS2a, and evaluate the photo-oxidative mechanism using reactive oxygen species (ROS) and lipid peroxidation probes combined with appropriate ROS scavengers. Light-induced intracellular redistribution of TPPS2a was observed, consistent with rupture of endolysosomes where the porphyrin localises. Using the same range of light doses, time-lapse confocal imaging permitted observation of PDT-induced generation of ROS in both 2D and 3D cancer models using fluorescence-based ROS together with specific ROS inhibitors. In addition, the use of red light excitation of the photosensitiser to minimise auto-oxidation of the probes was investigated. In the second part of the study, the photophysical properties of TPPS2a in cells were studied using a time-domain FLIM system with time-correlated single photon counting detection. Owing to the high sensitivity and spatial resolution of this system, we acquired FLIM images that enabled the fluorescence lifetime determination of the porphyrin within the endolysosomal vesicles. Changes in the lifetime dynamics upon prolonged illumination were revealed as the vesicles degraded within the cells.
Keywords: fluorescence lifetime imaging microscopy; photodynamic therapy; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Therapeutic enhancement of a cytotoxic agent using photochemical internalisation in 3D compressed collagen constructs of ovarian cancer.Acta Biomater. 2018 Nov;81:80-92. doi: 10.1016/j.actbio.2018.09.041. Epub 2018 Sep 27. Acta Biomater. 2018. PMID: 30267880
-
Efficacy of photochemical internalisation using disulfonated chlorin and porphyrin photosensitisers: An in vitro study in 2D and 3D prostate cancer models.Cancer Lett. 2017 May 1;393:68-75. doi: 10.1016/j.canlet.2017.02.018. Epub 2017 Feb 20. Cancer Lett. 2017. PMID: 28223166 Free PMC article.
-
Specific photodamage on HT-29 cancer cells leads to endolysosomal failure and autophagy blockage by cathepsin depletion.J Photochem Photobiol B. 2024 Jun;255:112919. doi: 10.1016/j.jphotobiol.2024.112919. Epub 2024 Apr 19. J Photochem Photobiol B. 2024. PMID: 38677261
-
Porphyrins and related macrocycles: Combining photosensitization with radio- or optical-imaging for next generation theranostic agents.Photodiagnosis Photodyn Ther. 2018 Sep;23:281-294. doi: 10.1016/j.pdpdt.2018.06.023. Epub 2018 Jul 29. Photodiagnosis Photodyn Ther. 2018. PMID: 30009949 Review.
-
Quinones as photosensitizer for photodynamic therapy: ROS generation, mechanism and detection methods.Photodiagnosis Photodyn Ther. 2016 Mar;13:175-187. doi: 10.1016/j.pdpdt.2015.07.177. Epub 2015 Aug 1. Photodiagnosis Photodyn Ther. 2016. PMID: 26241780 Review.
References
-
- Dos Santos A.F., De Almeida D.R.Q., Terra L.F., Baptista M.S., Labriola L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019;5:25. doi: 10.20517/2394-4722.2018.83. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

