Precise Targeting of Autoantigen-Specific B Cells in Lupus Nephritis with Chimeric Autoantibody Receptor T Cells
- PMID: 38673811
- PMCID: PMC11050013
- DOI: 10.3390/ijms25084226
Precise Targeting of Autoantigen-Specific B Cells in Lupus Nephritis with Chimeric Autoantibody Receptor T Cells
Abstract
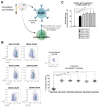
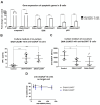
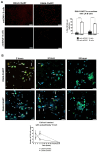
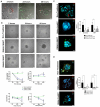
Despite conventional therapy, lupus nephritis (LN) remains a significant contributor to short- and long-term morbidity and mortality. B cell abnormalities and the production of autoantibodies against nuclear complexes like anti-dsDNA are recognised as key players in the pathogenesis of LN. To address the challenges of chronic immunosuppression associated with current therapies, we have engineered T cells to express chimeric autoantibody receptors (DNA-CAART) for the precise targeting of B cells expressing anti-dsDNA autoantibodies. T cells from LN patients were transduced using six different CAAR vectors based on their antigen specificity, including alpha-actinin, histone-1, heparan sulphate, or C1q. The cytotoxicity, cytokine production, and cell-cell contact of DNA-CAART were thoroughly investigated in co-culture experiments with B cells isolated from patients, both with and without anti-dsDNA positivity. The therapeutic effects were further evaluated using an in vitro immune kidney LN organoid. Among the six proposed DNA-CAART, DNA4 and DNA6 demonstrated superior selectively cytotoxic activity against anti-dsDNA+ B cells. Notably, DNA4-CAART exhibited improvements in organoid morphology, apoptosis, and the inflammatory process in the presence of IFNα-stimulated anti-dsDNA+ B cells. Based on these findings, DNA4-CAART emerge as promising candidates for modulating autoimmunity and represent a novel approach for the treatment of LN.
Keywords: B cell; CAART; anti-dsDNA; lupus nephritis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

