In Vivo Biocompatibility Study on Functional Nanostructures Containing Bioactive Glass and Plant Extracts for Implantology
- PMID: 38673834
- PMCID: PMC11050673
- DOI: 10.3390/ijms25084249
In Vivo Biocompatibility Study on Functional Nanostructures Containing Bioactive Glass and Plant Extracts for Implantology
Abstract
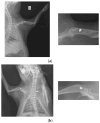
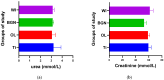
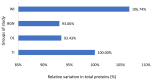
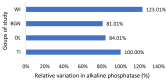
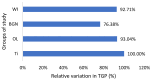
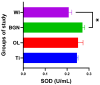
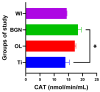
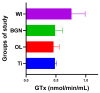
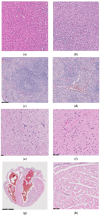
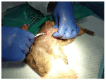
In this paper, the in vivo behavior of orthopedic implants covered with thin films obtained by matrix-assisted pulsed laser evaporation and containing bioactive glass, a polymer, and natural plant extract was evaluated. In vivo testing was performed by carrying out a study on guinea pigs who had coated metallic screws inserted in them and also controls, following the regulations of European laws regarding the use of animals in scientific studies. After 26 weeks from implantation, the guinea pigs were subjected to X-ray analyses to observe the evolution of osteointegration over time; the guinea pigs' blood was collected for the detection of enzymatic activity and to measure values for urea, creatinine, blood glucose, alkaline phosphatase, pancreatic amylase, total protein, and glutamate pyruvate transaminase to see the extent to which the body was affected by the introduction of the implant. Moreover, a histopathological assessment of the following vital organs was carried out: heart, brain, liver, and spleen. We also assessed implanted bone with adjacent tissue. Our studies did not find significant variations in biochemical and histological results compared to the control group or significant adverse effects caused by the implant coating in terms of tissue compatibility, inflammatory reactions, and systemic effects.
Keywords: in vivo study; orthopedic implant; plant extract.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



Similar articles
-
An evaluation of the biocompatibility and osseointegration of novel glass fiber reinforced composite implants: In vitro and in vivo studies.Dent Mater. 2018 Mar;34(3):470-485. doi: 10.1016/j.dental.2017.12.001. Epub 2017 Dec 26. Dent Mater. 2018. PMID: 29287979
-
Evaluation of bioactive glass for mastoid obliteration: a guinea pig model.In Vivo. 2007 Jul-Aug;21(4):651-5. In Vivo. 2007. PMID: 17708361
-
Enhanced biocompatibility and osseointegration of calcium titanate coating on titanium screws in rabbit femur.J Huazhong Univ Sci Technolog Med Sci. 2017 Jun;37(3):362-370. doi: 10.1007/s11596-017-1741-9. Epub 2017 Jun 6. J Huazhong Univ Sci Technolog Med Sci. 2017. PMID: 28585129
-
Fiber glass-bioactive glass composite for bone replacing and bone anchoring implants.Dent Mater. 2015 Apr;31(4):371-81. doi: 10.1016/j.dental.2015.01.003. Epub 2015 Jan 29. Dent Mater. 2015. PMID: 25640687 Review.
-
Strontium in the bone-implant interface.Dan Med Bull. 2011 May;58(5):B4286. Dan Med Bull. 2011. PMID: 21535993 Review.
Cited by
-
MAPLE-prepared graphene oxide-based coatings for improved orthopedic screws used in knee interventions.Rom J Morphol Embryol. 2024 Jul-Sep;65(3):433-442. doi: 10.47162/RJME.65.3.05. Rom J Morphol Embryol. 2024. PMID: 39529336 Free PMC article.
-
Integrating an antimicrobial nanocomposite to bioactive electrospun fibers for improved wound dressing materials.Sci Rep. 2024 Oct 24;14(1):25118. doi: 10.1038/s41598-024-75814-2. Sci Rep. 2024. PMID: 39443526 Free PMC article.
References
-
- Vladescu A., Badea M., Padmanabhan S.C., Paraschiv G., Floroian L., Gaman L., Morris M.A., Marty J.L., Cotrut C.M. Nanomaterials for medical applications and their antimicrobial advantages. In: Holban A.-M., Grumezescu A.M., editors. Materials for Biomedical Engineering. Elsevier Inc.; Amsterdam, The Netherlands: 2019. pp. 409–431.
-
- Hussain M., Askari Rizvi S.H., Abbas N., Sajjad U., Shad M.R., Badshah M.A., Malik A.I. Recent Developments in Coatings for Orthopedic Metallic Implants. Coatings. 2021;11:791. doi: 10.3390/coatings11070791. - DOI
-
- Floroian L., Ristoscu C., Mihailescu N., Negut I., Badea M., Ursutiu D., Chifiriuc M.C., Urzica I., Dyia H.M., Bleotu C., et al. Functionalized antimicrobial composite thin films printing for stainless steel implant coatings. Molecules. 2016;21:740. doi: 10.3390/molecules21060740. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

