Intraphagosomal Free Ca2+ Changes during Phagocytosis
- PMID: 38673839
- PMCID: PMC11050620
- DOI: 10.3390/ijms25084254
Intraphagosomal Free Ca2+ Changes during Phagocytosis
Abstract
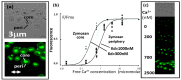
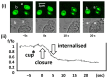
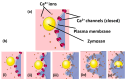
Phagocytosis (and endocytosis) is an unusual cellular process that results in the formation of a novel subcellular organelle, the phagosome. This phagosome contains not only the internalised target of phagocytosis but also the external medium, creating a new border between extracellular and intracellular environments. The boundary at the plasma membrane is, of course, tightly controlled and exploited in ionic cell signalling events. Although there has been much work on the control of phagocytosis by ions, notably, Ca2+ ions influxing across the plasma membrane, increasing our understanding of the mechanism enormously, very little work has been done exploring the phagosome/cytosol boundary. In this paper, we explored the changes in the intra-phagosomal Ca2+ ion content that occur during phagocytosis and phagosome formation in human neutrophils. Measuring Ca2+ ion concentration in the phagosome is potentially prone to artefacts as the intra-phagosomal environment experiences changes in pH and oxidation. However, by excluding such artefacts, we conclude that there are open Ca2+ channels on the phagosome that allow Ca2+ ions to "drain" into the surrounding cytosol. This conclusion was confirmed by monitoring the translocation of the intracellularly expressed YFP-tagged C2 domain of PKC-γ. This approach marked regions of membrane at which Ca2+ influx occurred, the earliest being the phagocytic cup, and then the whole cell. This paper therefore presents data that have novel implications for understanding phagocytic Ca2+ signalling events, such as peri-phagosomal Ca2+ hotspots, and other phenomena.
Keywords: Ca2+ channels; intra-phagosomal Ca2+; neutrophil; phagocytosis; phagosome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

