Investigating the Impact of Electrostatic Interactions on Calmodulin Binding and Ca2+-Dependent Activation of the Calcium-Gated Potassium SK4 Channel
- PMID: 38673845
- PMCID: PMC11050286
- DOI: 10.3390/ijms25084255
Investigating the Impact of Electrostatic Interactions on Calmodulin Binding and Ca2+-Dependent Activation of the Calcium-Gated Potassium SK4 Channel
Abstract
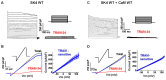
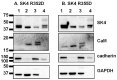
Ca2+ binding to the ubiquitous Ca2+ sensing protein calmodulin (CaM) activates the intermediate conductance Ca2+-activated SK4 channel. Potential hydrophilic pockets for CaM binding have been identified at the intracellular HA and HB helices in the C-terminal of SK4 from the three published cryo-EM structures of SK4. Single charge reversal substitutions at either site, significantly weakened the pull-down of SK4 by CaM wild-type (CaM), and decreased the TRAM-34 sensitive outward K+ current densities in native HEK293T cells when compared with SK4 WT measured under the same conditions. Only the doubly substituted SK4 R352D/R355D (HB helix) obliterated the CaM-mediated pull-down and thwarted outward K+ currents. However, overexpression of CaM E84K/E87K, which had been predicted to face the arginine doublet, restored the CaM-mediated pull-down of SK4 R352D/R355D and normalized its whole-cell current density. Virtual analysis of the putative salt bridges supports a unique role for the positively charged arginine doublet at the HB helix into anchoring the interaction with the negatively charged CaM glutamate 84 and 87 CaM. Our findings underscore the unique contribution of electrostatic interactions in carrying CaM binding onto SK4 and support the role of the C-terminal HB helix to the Ca2+-dependent gating process.
Keywords: calcium; calmodulin; co-immunoprecipitation; electrophysiology; ion channel; protein–protein interaction.
Conflict of interest statement
The authors declare no conflicts of interest with the content of the manuscript. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
Contribution of the KCa3.1 channel-calmodulin interactions to the regulation of the KCa3.1 gating process.J Gen Physiol. 2013 Jul;142(1):37-60. doi: 10.1085/jgp.201210933. J Gen Physiol. 2013. PMID: 23797421 Free PMC article.
-
Allosteric inhibitors targeting the calmodulin-PIP2 interface of SK4 K+ channels for atrial fibrillation treatment.Proc Natl Acad Sci U S A. 2022 Aug 23;119(34):e2202926119. doi: 10.1073/pnas.2202926119. Epub 2022 Aug 15. Proc Natl Acad Sci U S A. 2022. PMID: 35969786 Free PMC article.
-
Calmodulin regulates assembly and trafficking of SK4/IK1 Ca2+-activated K+ channels.J Biol Chem. 2001 Oct 12;276(41):37980-5. doi: 10.1074/jbc.M104965200. Epub 2001 Aug 8. J Biol Chem. 2001. PMID: 11495911
-
Calcium Role in Gap Junction Channel Gating: Direct Electrostatic or Calmodulin-Mediated?Int J Mol Sci. 2024 Sep 10;25(18):9789. doi: 10.3390/ijms25189789. Int J Mol Sci. 2024. PMID: 39337278 Free PMC article. Review.
-
Atomistic Insights of Calmodulin Gating of Complete Ion Channels.Int J Mol Sci. 2020 Feb 14;21(4):1285. doi: 10.3390/ijms21041285. Int J Mol Sci. 2020. PMID: 32075037 Free PMC article. Review.
References
-
- Gardos G. Effect of ethylenediaminetetraacetate on the permeability of human erythrocytes. Acta Physiol. Acad. Sci. Hung. 1958;14:1–5. - PubMed
-
- Skibsbye L., Poulet C., Diness J.G., Bentzen B.H., Yuan L., Kappert U., Matschke K., Wettwer E., Ravens U., Grunnet M., et al. Small-conductance calcium-activated potassium (SK) channels contribute to action potential repolarization in human atria. Cardiovasc. Res. 2014;103:156–167. doi: 10.1093/cvr/cvu121. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

