Novel Fe3O4 Nanoparticles with Bioactive Glass-Naproxen Coating: Synthesis, Characterization, and In Vitro Evaluation of Bioactivity
- PMID: 38673856
- PMCID: PMC11049812
- DOI: 10.3390/ijms25084270
Novel Fe3O4 Nanoparticles with Bioactive Glass-Naproxen Coating: Synthesis, Characterization, and In Vitro Evaluation of Bioactivity
Abstract
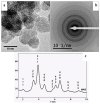
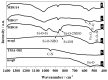
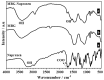
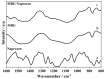
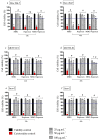
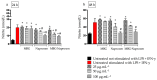
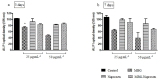
Immune response to biomaterials, which is intimately related to their surface properties, can produce chronic inflammation and fibrosis, leading to implant failure. This study investigated the development of magnetic nanoparticles coated with silica and incorporating the anti-inflammatory drug naproxen, aimed at multifunctional biomedical applications. The synthesized nanoparticles were characterized using various techniques that confirmed the presence of magnetite and the formation of a silica-rich bioactive glass (BG) layer. In vitro studies demonstrated that the nanoparticles exhibited bioactive properties, forming an apatite surface layer when immersed in simulated body fluid, and biocompatibility with bone cells, with good viability and alkaline phosphatase activity. Naproxen, either free or encapsulated, reduced nitric oxide production, an inflammatory marker, while the BG coating alone did not show anti-inflammatory effects in this study. Overall, the magnetic nanoparticles coated with BG and naproxen showed promise for biomedical applications, especially anti-inflammatory activity in macrophages and in the bone field, due to their biocompatibility, bioactivity, and osteogenic potential.
Keywords: biocompatibility; magnetic nanoparticles; naproxen; silica.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Bioactive glass (45S5)-based 3D scaffolds coated with magnesium and zinc-loaded hydroxyapatite nanoparticles for tissue engineering applications.Colloids Surf B Biointerfaces. 2019 Oct 1;182:110346. doi: 10.1016/j.colsurfb.2019.110346. Epub 2019 Jul 4. Colloids Surf B Biointerfaces. 2019. PMID: 31325780
-
Biocompatibility assessment of sub-5 nm silica-coated superparamagnetic iron oxide nanoparticles in human stem cells and in mice for potential application in nanomedicine.Nanoscale. 2020 Jan 23;12(3):1759-1778. doi: 10.1039/c9nr09683c. Nanoscale. 2020. PMID: 31895375
-
Cellulose Nanocrystals--Bioactive Glass Hybrid Coating as Bone Substitutes by Electrophoretic Co-deposition: In Situ Control of Mineralization of Bioactive Glass and Enhancement of Osteoblastic Performance.ACS Appl Mater Interfaces. 2015 Nov 11;7(44):24715-25. doi: 10.1021/acsami.5b07294. Epub 2015 Oct 27. ACS Appl Mater Interfaces. 2015. PMID: 26460819
-
Peptide-laden mesoporous silica nanoparticles with promoted bioactivity and osteo-differentiation ability for bone tissue engineering.Colloids Surf B Biointerfaces. 2015 Jul 1;131:73-82. doi: 10.1016/j.colsurfb.2015.04.043. Epub 2015 Apr 29. Colloids Surf B Biointerfaces. 2015. PMID: 25969416
-
Multifunctional bioactive glass nanoparticles: surface-interface decoration and biomedical applications.Regen Biomater. 2024 Sep 6;11:rbae110. doi: 10.1093/rb/rbae110. eCollection 2024. Regen Biomater. 2024. PMID: 39323748 Free PMC article. Review.
Cited by
-
Development of Scaffolds with Chitosan Magnetically Activated with Cobalt Nanoferrite: A Study on Physical-Chemical, Mechanical, Cytotoxic and Antimicrobial Behavior.Pharmaceuticals (Basel). 2024 Oct 5;17(10):1332. doi: 10.3390/ph17101332. Pharmaceuticals (Basel). 2024. PMID: 39458973 Free PMC article.
References
-
- Perez-Pariente J., Balas F., Vallet-Reg M. Surface and chemical study of SiO2 • P2O5 • CaO • (MgO) bioactive glasses. Chem. Mater. 2000;12:750–755. doi: 10.1021/cm9911114. - DOI
MeSH terms
Substances
Grants and funding
- APQ-03585-16/Fundação de Amparo à Pesquisa do Estado de Minas Gerais
- 01/2024/Universidade Federal de Ouro Preto
- 202212/2007-6/National Council for Scientific and Technological Development
- 404938/2016-7/National Council for Scientific and Technological Development
- 4465656/2014-5/Institutos Nacionais de Ciência e Tecnologia
- RED00079-22/Rede Mineira de Nanomedicina Teranóstica (FAPEMIG)
- RED-00570- 16/Rede Mineira de Engenharia de Tecidos e Terapia Celular (REMETTEC/FAPEMIG)
- UIDB/50011/2020/CICECO-Aveiro Institute of Materials
- UIDP/50011/2020/CICECO-Aveiro Institute of Materials
- LA/P/0006/2020/CICECO-Aveiro Institute of Materials
LinkOut - more resources
Full Text Sources

