Cardioprotection and Suppression of Fibrosis by Diverse Cancer and Non-Cancer Cell Lines in a Murine Model of Duchenne Muscular Dystrophy
- PMID: 38673859
- PMCID: PMC11050177
- DOI: 10.3390/ijms25084273
Cardioprotection and Suppression of Fibrosis by Diverse Cancer and Non-Cancer Cell Lines in a Murine Model of Duchenne Muscular Dystrophy
Abstract
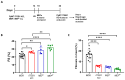
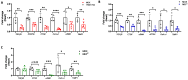
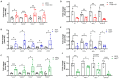
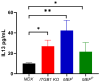
The dynamic relationship between heart failure and cancer poses a dual challenge. While cardiac remodeling can promote cancer growth and metastasis, tumor development can ameliorate cardiac dysfunction and suppress fibrosis. However, the precise mechanism through which cancer influences the heart and fibrosis is yet to be uncovered. To further explore the interaction between heart failure and cancer, we used the MDX mouse model, which suffers from cardiac fibrosis and cardiac dysfunction. A previous study from our lab demonstrated that tumor growth improves cardiac dysfunction and dampens fibrosis in the heart and diaphragm muscles of MDX mice. We used breast Polyoma middle T (PyMT) and Lewis lung carcinoma (LLC) cancer cell lines that developed into large tumors. To explore whether the aggressiveness of the cancer cell line is crucial for the beneficial phenotype, we employed a PyMT breast cancer cell line lacking integrin β1, representing a less aggressive cell line compared to the original PyMT cells. In addition, we examined immortalized and primary MEF cells. The injection of integrin β1 KO PyMT cancer cells and Mouse Embryo Fibroblasts cells (MEF) resulted in the improvement of cardiac function and decreased fibrosis in the heart, diaphragm, and skeletal muscles of MDX mice. Collectively, our data demonstrate that the cancer line aggressiveness as well as primary MEF cells are sufficient to impose the beneficial phenotype. These discoveries present potential novel clinical therapeutic approaches with beneficial outcome for patients with fibrotic diseases and cardiac dysfunction that do not require tumor growth.
Keywords: Duchenne muscular dystrophy; cardiac dysfunction; cardiac remodeling; fibrosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Tumor Growth Ameliorates Cardiac Dysfunction and Suppresses Fibrosis in a Mouse Model for Duchenne Muscular Dystrophy.Int J Mol Sci. 2023 Aug 9;24(16):12595. doi: 10.3390/ijms241612595. Int J Mol Sci. 2023. PMID: 37628775 Free PMC article.
-
Skeletal muscle fibrosis in the mdx/utrn+/- mouse validates its suitability as a murine model of Duchenne muscular dystrophy.PLoS One. 2015 Jan 21;10(1):e0117306. doi: 10.1371/journal.pone.0117306. eCollection 2015. PLoS One. 2015. PMID: 25607927 Free PMC article.
-
Exclusive skeletal muscle correction does not modulate dystrophic heart disease in the aged mdx model of Duchenne cardiomyopathy.Hum Mol Genet. 2013 Jul 1;22(13):2634-41. doi: 10.1093/hmg/ddt112. Epub 2013 Mar 3. Hum Mol Genet. 2013. PMID: 23459935 Free PMC article.
-
Diaphragm muscle fibrosis involves changes in collagen organization with mechanical implications in Duchenne muscular dystrophy.J Appl Physiol (1985). 2022 Mar 1;132(3):653-672. doi: 10.1152/japplphysiol.00248.2021. Epub 2022 Jan 20. J Appl Physiol (1985). 2022. PMID: 35050792 Free PMC article.
-
Murine models of Duchenne muscular dystrophy: is there a best model?Am J Physiol Cell Physiol. 2021 Aug 1;321(2):C409-C412. doi: 10.1152/ajpcell.00212.2021. Epub 2021 Jul 14. Am J Physiol Cell Physiol. 2021. PMID: 34260298 Review. No abstract available.
References
-
- Giovarelli M., Arnaboldi F., Zecchini S., Cornaghi L.B., Nava A., Sommariva M., Clementi E.G.I., Gagliano N. Characterisation of progressive skeletal muscle fibrosis in the Mdx mouse model of duchenne muscular dystrophy: An in vivo and in vitro study. Int. J. Mol. Sci. 2022;23:8735. doi: 10.3390/ijms23158735. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

