Development of a Novel, Potent, and Selective Sialyltransferase Inhibitor for Suppressing Cancer Metastasis
- PMID: 38673867
- PMCID: PMC11050067
- DOI: 10.3390/ijms25084283
Development of a Novel, Potent, and Selective Sialyltransferase Inhibitor for Suppressing Cancer Metastasis
Abstract
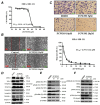
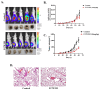
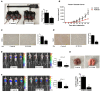
Sialyltransferase-catalyzed membrane protein and lipid glycosylation plays a vital role as one of the most abundant post-translational modifications and diversification reactions in eukaryotes. However, aberrant sialylation has been associated with cancer malignancy and metastasis. Sialyltransferases thus represent emerging targets for the development of small molecule cancer drugs. Herein, we report the inhibitory effects of a recently discovered lithocholic acid derivative FCW393 on sialyltransferase catalytic activity, integrin sialyation, cancer-associated signal transduction, MDA-MB-231 and B16F10 cell migration and invasion, and in in vivo studies, on tumor growth, metastasis, and angiogenesis. FCW393 showed effective and selective inhibition of the sialyltransferases ST6GAL1 (IC50 = 7.8 μM) and ST3GAL3 (IC50 = 9.45 μM) relative to ST3GAL1 (IC50 > 400 μM) and ST8SIA4 (IC50 > 100 μM). FCW393 reduced integrin sialylation in breast cancer and melanoma cells dose-dependently and downregulated proteins associated with the integrin-regulated FAK/paxillin and GEF/Rho/ROCK pathways, and with the VEGF-regulated Akt/NFκB/HIF-1α pathway. FCW393 inhibited cell migration (IC50 = 2.6 μM) and invasion in in vitro experiments, and in in vivo studies of tumor-bearing mice, FCW393 reduced tumor size, angiogenesis, and metastatic potential. Based on its demonstrated selectivity, cell permeability, relatively low cytotoxicity (IC50 = 55 μM), and high efficacy, FCW393 shows promising potential as a small molecule experimental tool compound and a lead for further development of a novel cancer therapeutic.
Keywords: breast cancer; integrin sialylation; melanoma; metastasis; sialyltransferase inhibitor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- AS-KPQ-110-EIMD, AS-KPQ-109-BioMed, AS-KPQ-110-BioMed and AS-KPQ-111-KNT/Academia Sinica
- MOST, Taiwan, MOST 110-0210-01-22-02, MOST-108-3114-Y-001-002, MOST 108-3111-Y-001-056, MOST 106-2113-M-001-011, MOST 103-2325-B-001-001 and MOST108-2314-B-110-003-MY2/Ministry of Science and Technology, TAIWAN
- 108-36/Kaohsiung Armed Forces General Hospital, TAIWAN
LinkOut - more resources
Full Text Sources
Miscellaneous

