Effects of Mild Closed-Head Injury and Subanesthetic Ketamine Infusion on Microglia, Axonal Injury, and Synaptic Density in Sprague-Dawley Rats
- PMID: 38673871
- PMCID: PMC11050690
- DOI: 10.3390/ijms25084287
Effects of Mild Closed-Head Injury and Subanesthetic Ketamine Infusion on Microglia, Axonal Injury, and Synaptic Density in Sprague-Dawley Rats
Abstract
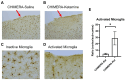
Mild traumatic brain injury (mTBI) affects millions of people in the U.S. Approximately 20-30% of those individuals develop adverse symptoms lasting at least 3 months. In a rat mTBI study, the closed-head impact model of engineered rotational acceleration (CHIMERA) produced significant axonal injury in the optic tract (OT), indicating white-matter damage. Because retinal ganglion cells project to the lateral geniculate nucleus (LGN) in the thalamus through the OT, we hypothesized that synaptic density may be reduced in the LGN of rats following CHIMERA injury. A modified SEQUIN (synaptic evaluation and quantification by imaging nanostructure) method, combined with immunofluorescent double-labeling of pre-synaptic (synapsin) and post-synaptic (PSD-95) markers, was used to quantify synaptic density in the LGN. Microglial activation at the CHIMERA injury site was determined using Iba-1 immunohistochemistry. Additionally, the effects of ketamine, a potential neuroprotective drug, were evaluated in CHIMERA-induced mTBI. A single-session repetitive (ssr-) CHIMERA (3 impacts, 1.5 joule/impact) produced mild effects on microglial activation at the injury site, which was significantly enhanced by post-injury intravenous ketamine (10 mg/kg) infusion. However, ssr-CHIMERA did not alter synaptic density in the LGN, although ketamine produced a trend of reduction in synaptic density at post-injury day 4. Further research is necessary to characterize the effects of ssr-CHIMERA and subanesthetic doses of intravenous ketamine on different brain regions and multiple time points post-injury. The current study demonstrates the utility of the ssr-CHIMERA as a rodent model of mTBI, which researchers can use to identify biological mechanisms of mTBI and to develop improved treatment strategies for individuals suffering from head trauma.
Keywords: axonal injury; ketamine; lateral geniculate nucleus; mild traumatic brain injury; rats; synaptic density.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- CDC . Report to Congress on Traumatic Brain Injury Epidemiology and Rehabilitation. CDC; Atlanta, GA, USA: 2015.
-
- Cancelliere C., Verville L., Stubbs J.L., Yu H., Hincapie C.A., Cassidy J.D., Wong J.J., Shearer H.M., Connell G., Southerst D., et al. Post-Concussion Symptoms and Disability in Adults with Mild Traumatic Brain Injury: A Systematic Review and Meta-Analysis. J. Neurotrauma. 2023;40:1045–1059. doi: 10.1089/neu.2022.0185. - DOI - PubMed
-
- Va/DoD . VA/DoD Clinical Practice Guideline for the Management and Rehabilitation of Post-Acute Mild Traumatic Brain Injury. Va/DoD; Washington, DC, USA: 2021.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

