Quercetin Impairs the Growth of Uveal Melanoma Cells by Interfering with Glucose Uptake and Metabolism
- PMID: 38673877
- PMCID: PMC11049862
- DOI: 10.3390/ijms25084292
Quercetin Impairs the Growth of Uveal Melanoma Cells by Interfering with Glucose Uptake and Metabolism
Abstract
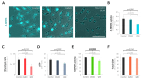
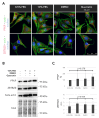
Monosomy 3 in uveal melanoma (UM) increases the risk of lethal metastases, mainly in the liver, which serves as the major site for the storage of excessive glucose and the metabolization of the dietary flavonoid quercetin. Although primary UMs with monosomy 3 exhibit a higher potential for basal glucose uptake, it remains unknown as to whether glycolytic capacity is altered in such tumors. Herein, we initially analyzed the expression of n = 151 genes involved in glycolysis and its interconnected branch, the "pentose phosphate pathway (PPP)", in the UM cohort of The Cancer Genome Atlas Study and validated the differentially expressed genes in two independent cohorts. We also evaluated the effects of quercetin on the growth, survival, and glucose metabolism of the UM cell line 92.1. The rate-limiting glycolytic enzyme PFKP was overexpressed whereas the ZBTB20 gene (locus: 3q13.31) was downregulated in the patients with metastases in all cohorts. Quercetin was able to impair proliferation, viability, glucose uptake, glycolysis, ATP synthesis, and PPP rate-limiting enzyme activity while increasing oxidative stress. UMs with monosomy 3 display a stronger potential to utilize glucose for the generation of energy and biomass. Quercetin can prevent the growth of UM cells by interfering with glucose metabolism.
Keywords: glucose uptake; glycolysis; oxidative stress; pentose phosphate pathway; proliferation; quercetin; uveal melanoma.
Conflict of interest statement
A.T. received financial support from Novartis Pharma, Germany. The funder had no role in the design of the study; in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results. The remaining authors declare no conflicts of interest.
Figures



Similar articles
-
Monosomy-3 Alters the Expression Profile of the Glucose Transporters GLUT1-3 in Uveal Melanoma.Int J Mol Sci. 2020 Dec 8;21(24):9345. doi: 10.3390/ijms21249345. Int J Mol Sci. 2020. PMID: 33302435 Free PMC article.
-
Monosomy 3 Is Linked to Resistance to MEK Inhibitors in Uveal Melanoma.Int J Mol Sci. 2021 Jun 23;22(13):6727. doi: 10.3390/ijms22136727. Int J Mol Sci. 2021. PMID: 34201614 Free PMC article.
-
Establishment and characterization of two uveal melanoma cell lines derived from tumors with loss of one chromosome 3.Exp Eye Res. 2006 Oct;83(4):858-64. doi: 10.1016/j.exer.2006.04.004. Epub 2006 Jun 5. Exp Eye Res. 2006. PMID: 16750193 Review.
-
The role of RASSF1A in uveal melanoma.Invest Ophthalmol Vis Sci. 2012 Oct 3;53(6):2611-9. doi: 10.1167/iovs.11-7730. Invest Ophthalmol Vis Sci. 2012. PMID: 22447862
-
Prognostication of uveal melanoma is simple and highly predictive using The Cancer Genome Atlas (TCGA) classification: A review.Indian J Ophthalmol. 2019 Dec;67(12):1959-1963. doi: 10.4103/ijo.IJO_1589_19. Indian J Ophthalmol. 2019. PMID: 31755428 Free PMC article. Review.
Cited by
-
Low-Dose Quercetin Dephosphorylates AKT and Suppresses Proteins Related to Migration in Human Metastatic Uveal Melanoma Cells.Life (Basel). 2025 Jun 18;15(6):979. doi: 10.3390/life15060979. Life (Basel). 2025. PMID: 40566630 Free PMC article.
-
[Circulating tumor cells in uveal melanoma : "The needle in the haystack"].Ophthalmologie. 2024 Dec;121(12):954-962. doi: 10.1007/s00347-024-02136-z. Epub 2024 Nov 23. Ophthalmologie. 2024. PMID: 39580374 Review. German.
References
-
- Blum E.S., Yang J., Komatsubara K.M., Carvajal R.D. Clinical Management of Uveal and Conjunctival Melanoma. Oncology. 2016;30 - PubMed
-
- Prescher G., Bornfeld N., Hirche H., Horsthemke B., Jöckel K.H., Becher R. Prognostic Implications of Monosomy 3 in Uveal Melanoma. Lancet. 1996;347:1222–1225. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

