MyoD Over-Expression Rescues GST-bFGF Repressed Myogenesis
- PMID: 38673893
- PMCID: PMC11050597
- DOI: 10.3390/ijms25084308
MyoD Over-Expression Rescues GST-bFGF Repressed Myogenesis
Abstract
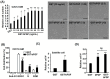
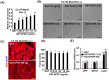
During embryogenesis, basic fibroblast growth factor (bFGF) is released from neural tube and myotome to promote myogenic fate in the somite, and is routinely used for the culture of adult skeletal muscle (SKM) stem cells (MuSC, called satellite cells). However, the mechanism employed by bFGF to promote SKM lineage and MuSC proliferation has not been analyzed in detail. Furthermore, the question of if the post-translational modification (PTM) of bFGF is important to its stemness-promoting effect has not been answered. In this study, GST-bFGF was expressed and purified from E.coli, which lacks the PTM system in eukaryotes. We found that both GST-bFGF and commercially available bFGF activated the Akt-Erk pathway and had strong cell proliferation effect on C2C12 myoblasts and MuSC. GST-bFGF reversibly compromised the myogenesis of C2C12 myoblasts and MuSC, and it increased the expression of Myf5, Pax3/7, and Cyclin D1 but strongly repressed that of MyoD, suggesting the maintenance of myogenic stemness amid repressed MyoD expression. The proliferation effect of GST-bFGF was conserved in C2C12 over-expressed with MyoD (C2C12-tTA-MyoD), implying its independence of the down-regulation of MyoD. In addition, the repressive effect of GST-bFGF on myogenic differentiation was almost totally rescued by the over-expression of MyoD. Together, these evidences suggest that (1) GST-bFGF and bFGF have similar effects on myogenic cell proliferation and differentiation, and (2) GST-bFGF can promote MuSC stemness and proliferation by differentially regulating MRFs and Pax3/7, (3) MyoD repression by GST-bFGF is reversible and independent of the proliferation effect, and (4) GST-bFGF can be a good substitute for bFGF in sustaining MuSC stemness and proliferation.
Keywords: MyoD; bFGF; cell cycle; differentiation; muscle; myogenesis.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





Similar articles
-
Cyclin D3 promotes myogenic differentiation and Pax7 transcription.J Cell Biochem. 2012 Jan;113(1):209-19. doi: 10.1002/jcb.23346. J Cell Biochem. 2012. PMID: 21898542
-
Post-transcriptional regulation of myogenic transcription factors during muscle development and pathogenesis.J Muscle Res Cell Motil. 2024 Mar;45(1):21-39. doi: 10.1007/s10974-023-09663-3. Epub 2024 Jan 11. J Muscle Res Cell Motil. 2024. PMID: 38206489 Review.
-
Signals and myogenic regulatory factors restrict pax3 and pax7 expression to dermomyotome-like tissue in zebrafish.Dev Biol. 2007 Feb 15;302(2):504-21. doi: 10.1016/j.ydbio.2006.10.009. Epub 2006 Oct 10. Dev Biol. 2007. PMID: 17094960 Free PMC article.
-
Alveolar rhabdomyosarcoma-associated proteins PAX3/FOXO1A and PAX7/FOXO1A suppress the transcriptional activity of MyoD-target genes in muscle stem cells.Oncogene. 2013 Jan 31;32(5):651-62. doi: 10.1038/onc.2012.73. Epub 2012 Jun 18. Oncogene. 2013. PMID: 22710712
-
Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis.Semin Cell Dev Biol. 2017 Dec;72:19-32. doi: 10.1016/j.semcdb.2017.11.011. Epub 2017 Nov 15. Semin Cell Dev Biol. 2017. PMID: 29127046 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

