Therapeutic Potential of 4-Hexylresorcinol in Preserving Testicular Function in Streptozotocin-Induced Diabetic Rats
- PMID: 38673900
- PMCID: PMC11050698
- DOI: 10.3390/ijms25084316
Therapeutic Potential of 4-Hexylresorcinol in Preserving Testicular Function in Streptozotocin-Induced Diabetic Rats
Abstract
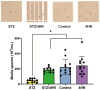
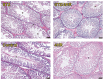
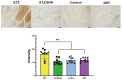
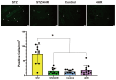
It is known that many diabetic patients experience testicular atrophy. This study sought to investigate the effect of 4-hexylresorcinol (4HR) on testicular function in rats with streptozotocin (STZ)-induced diabetes, focusing on testicular weight, sperm motility, histological alterations, and serum testosterone levels to understand the efficacy of 4HR on testes. Our findings reveal that 4HR treatment significantly improves testicular health in diabetic rats. Notably, the STZ group exhibited a testicular weight of 1.22 ± 0.48 g, whereas the STZ/4HR group showed a significantly enhanced weight of 1.91 ± 0.26 g (p < 0.001), aligning closely with the control group's weight of 1.99 ± 0.17 g and the 4HR group's weight of 2.05 ± 0.24 g, indicating no significant difference between control and 4HR groups (p > 0.05). Furthermore, the STZ/4HR group demonstrated significantly improved sperm motility compared to the STZ group, with apoptotic indicators notably reduced in the STZ/4HR group relative to the STZ group (p < 0.05). These results underscore the therapeutic potential of 4HR for maintaining testicular function under diabetic conditions.
Keywords: 4-hexylresorcinol; apoptosis; diabetes; testis; testosterone.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Protective effects of nanostructures of hydrated C(60) fullerene on reproductive function in streptozotocin-diabetic male rats.Toxicology. 2011 Apr 11;282(3):69-81. doi: 10.1016/j.tox.2010.12.003. Epub 2010 Dec 14. Toxicology. 2011. PMID: 21163323
-
Effects of 4-hexylresorcinol on facial skeletal development in growing rats: Considerations for diabetes.Korean J Orthod. 2023 Nov 25;53(6):393-401. doi: 10.4041/kjod23.091. Epub 2023 Sep 5. Korean J Orthod. 2023. PMID: 37989576 Free PMC article.
-
Ferulic acid in the treatment of post-diabetes testicular damage: relevance to the down regulation of apoptosis correlates with antioxidant status via modulation of TGF-β1, IL-1β and Akt signalling.Cell Biochem Funct. 2014 Jan;32(1):115-24. doi: 10.1002/cbf.2983. Epub 2013 May 10. Cell Biochem Funct. 2014. PMID: 23661600
-
Icariin protects testicular damage in streptozotocin-induced diabetic rats through regulation of glycolysis pathway.Int J Immunopathol Pharmacol. 2024 Jan-Dec;38:3946320241279525. doi: 10.1177/03946320241279525. Int J Immunopathol Pharmacol. 2024. PMID: 39180223 Free PMC article.
-
Effects of Vernonia cinerea on reproductive performance in streptozotocin-induced diabetic rats.J Vet Med Sci. 2017 Mar 23;79(3):572-578. doi: 10.1292/jvms.16-0466. Epub 2017 Feb 10. J Vet Med Sci. 2017. PMID: 28190818 Free PMC article.

