Stereoselective Synthesis and Catalytical Application of Perillaldehyde-Based 3-Amino-1,2-diol Regioisomers
- PMID: 38673908
- PMCID: PMC11050431
- DOI: 10.3390/ijms25084325
Stereoselective Synthesis and Catalytical Application of Perillaldehyde-Based 3-Amino-1,2-diol Regioisomers
Abstract
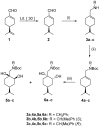
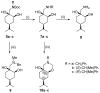
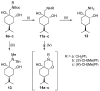
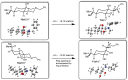
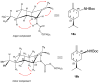
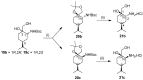
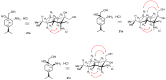
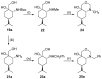
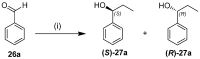
A library of regioisomeric monoterpene-based aminodiols was synthesised and applied as chiral catalysts in the addition of diethylzinc to benzaldehyde. The synthesis of the first type of aminodiols was achieved starting from (-)-8,9-dihydroperillaldehyde via reductive amination, followed by Boc protection and dihydroxylation with the OsO4/NMO system. Separation of formed stereoisomers resulted in a library of aminodiol diastereoisomers. The library of regioisomeric analogues was obtained starting from (-)-8,9-dihydroperillic alcohol, which was transformed into a mixture of allylic trichloroacetamides via Overman rearrangement. Changing the protecting group to a Boc function, the protected enamines were subjected to dihydroxylation with the OsO4/NMO system, leading to a 71:16:13 mixture of diastereoisomers, which were separated, affording the three isomers in isolated form. The obtained primary aminodiols were transformed into secondary derivatives. The regioselectivity of the ring closure of the N-benzyl-substituted aminodiols with formaldehyde was also investigated, resulting in 1,3-oxazines in an exclusive manner. To explain the stability difference between diastereoisomeric 1,3-oxazines, a series of comparative theoretical modelling studies was carried out. The obtained potential catalysts were applied in the reaction of aromatic aldehydes and diethylzinc with moderate to good enantioselectivities (up to 94% ee), whereas the opposite chiral selectivity was observed between secondary aminodiols and their ring-closed 1,3-oxazine analogues.
Keywords: aminodiol; catalyst; chiral; diethylzinc; enantioselective; monoterpene; regioselective.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
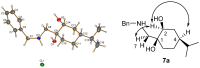



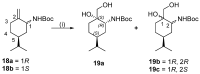
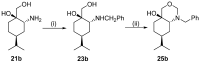

Similar articles
-
Stereoselective Synthesis and Modelling-Driven Optimisation of Carane-Based Aminodiols and 1,3-Oxazines as Catalysts for the Enantioselective Addition of Diethylzinc to Benzaldehyde.Chemistry. 2016 May 17;22(21):7163-73. doi: 10.1002/chem.201600749. Epub 2016 Apr 13. Chemistry. 2016. PMID: 27072603
-
Stereoselective Synthesis and Investigation of Isopulegol-Based Chiral Ligands.Int J Mol Sci. 2019 Aug 19;20(16):4050. doi: 10.3390/ijms20164050. Int J Mol Sci. 2019. PMID: 31430981 Free PMC article.
-
Stereoselective Syntheses and Application of Chiral Bi- and Tridentate Ligands Derived from (+)-Sabinol.Molecules. 2018 Mar 27;23(4):771. doi: 10.3390/molecules23040771. Molecules. 2018. PMID: 29584708 Free PMC article.
-
Catalytic asymmetric organozinc additions to carbonyl compounds.Chem Rev. 2001 Mar;101(3):757-824. doi: 10.1021/cr000411y. Chem Rev. 2001. PMID: 11712502 Review.
-
Asymmetric functional organozinc additions to aldehydes catalyzed by 1,1'-bi-2-naphthols (BINOLs).Acc Chem Res. 2014 May 20;47(5):1523-35. doi: 10.1021/ar500020k. Epub 2014 Apr 16. Acc Chem Res. 2014. PMID: 24738985 Free PMC article. Review.
Cited by
-
Biological Activity of Monoterpene-Based Scaffolds: A Natural Toolbox for Drug Discovery.Molecules. 2025 Mar 27;30(7):1480. doi: 10.3390/molecules30071480. Molecules. 2025. PMID: 40286078 Free PMC article. Review.
References
-
- Berkessel A., Gröger H. Asymmetric Organocatalysis—From Biomimetic Concepts to Applications in Asymmetric Synthesis. Wiley-VCH; Weinheim, Germany: 2005.
-
- Lin G.-Q., You Q.-D., Cheng J.-F. Chiral Drugs: Chemistry and Biological Action. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2011.
-
- Liu Q., Xie X., Tang M., Tao W., Shi T., Zhang Y., Huang T., Zhao Y., Deng Z., Lin S. One-Pot Asymmetric Synthesis of an Aminodiol Intermediate of Florfenicol Using Engineered Transketolase and Transaminase. ACS Catal. 2021;11:7477–7488. doi: 10.1021/acscatal.1c01229. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

