Molecular Pathways of Vulnerable Carotid Plaques at Risk of Ischemic Stroke: A Narrative Review
- PMID: 38673936
- PMCID: PMC11050267
- DOI: 10.3390/ijms25084351
Molecular Pathways of Vulnerable Carotid Plaques at Risk of Ischemic Stroke: A Narrative Review
Abstract
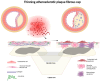
The concept of vulnerable carotid plaques is pivotal in understanding the pathophysiology of ischemic stroke secondary to large-artery atherosclerosis. In macroscopic evaluation, vulnerable plaques are characterized by one or more of the following features: microcalcification; neovascularization; lipid-rich necrotic cores (LRNCs); intraplaque hemorrhage (IPH); thin fibrous caps; plaque surface ulceration; huge dimensions, suggesting stenosis; and plaque rupture. Recognizing these macroscopic characteristics is crucial for estimating the risk of cerebrovascular events, also in the case of non-significant (less than 50%) stenosis. Inflammatory biomarkers, such as cytokines and adhesion molecules, lipid-related markers like oxidized low-density lipoprotein (LDL), and proteolytic enzymes capable of degrading extracellular matrix components are among the key molecules that are scrutinized for their associative roles in plaque instability. Through their quantification and evaluation, these biomarkers reveal intricate molecular cross-talk governing plaque inflammation, rupture potential, and thrombogenicity. The current evidence demonstrates that plaque vulnerability phenotypes are multiple and heterogeneous and are associated with many highly complex molecular pathways that determine the activation of an immune-mediated cascade that culminates in thromboinflammation. This narrative review provides a comprehensive analysis of the current knowledge on molecular biomarkers expressed by symptomatic carotid plaques. It explores the association of these biomarkers with the structural and compositional attributes that characterize vulnerable plaques.
Keywords: carotid plaque; inflammation; stroke; vulnerability.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

