AAV-Mediated CAG-Targeting Selectively Reduces Polyglutamine-Expanded Protein and Attenuates Disease Phenotypes in a Spinocerebellar Ataxia Mouse Model
- PMID: 38673939
- PMCID: PMC11050704
- DOI: 10.3390/ijms25084354
AAV-Mediated CAG-Targeting Selectively Reduces Polyglutamine-Expanded Protein and Attenuates Disease Phenotypes in a Spinocerebellar Ataxia Mouse Model
Abstract
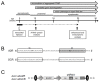
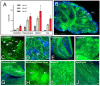
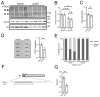
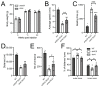
Polyglutamine (polyQ)-encoding CAG repeat expansions represent a common disease-causing mutation responsible for several dominant spinocerebellar ataxias (SCAs). PolyQ-expanded SCA proteins are toxic for cerebellar neurons, with Purkinje cells (PCs) being the most vulnerable. RNA interference (RNAi) reagents targeting transcripts with expanded CAG reduce the level of various mutant SCA proteins in an allele-selective manner in vitro and represent promising universal tools for treating multiple CAG/polyQ SCAs. However, it remains unclear whether the therapeutic targeting of CAG expansion can be achieved in vivo and if it can ameliorate cerebellar functions. Here, using a mouse model of SCA7 expressing a mutant Atxn7 allele with 140 CAGs, we examined the efficacy of short hairpin RNAs (shRNAs) targeting CAG repeats expressed from PHP.eB adeno-associated virus vectors (AAVs), which were introduced into the brain via intravascular injection. We demonstrated that shRNAs carrying various mismatches with the CAG target sequence reduced the level of polyQ-expanded ATXN7 in the cerebellum, albeit with varying degrees of allele selectivity and safety profile. An shRNA named A4 potently reduced the level of polyQ-expanded ATXN7, with no effect on normal ATXN7 levels and no adverse side effects. Furthermore, A4 shRNA treatment improved a range of motor and behavioral parameters 23 weeks after AAV injection and attenuated the disease burden of PCs by preventing the downregulation of several PC-type-specific genes. Our results show the feasibility of the selective targeting of CAG expansion in the cerebellum using a blood-brain barrier-permeable vector to attenuate the disease phenotype in an SCA mouse model. Our study represents a significant advancement in developing CAG-targeting strategies as a potential therapy for SCA7 and possibly other CAG/polyQ SCAs.
Keywords: CAG repeat expansion; CAG-targeting therapy; Spinocerebellar ataxia; adeno-associated virus; allele selectivity; short hairpin RNA.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Molecular Targets and Therapeutic Strategies in Spinocerebellar Ataxia Type 7.Neurotherapeutics. 2019 Oct;16(4):1074-1096. doi: 10.1007/s13311-019-00778-5. Neurotherapeutics. 2019. PMID: 31432449 Free PMC article. Review.
-
Reduction of mutant ataxin-7 expression restores motor function and prevents cerebellar synaptic reorganization in a conditional mouse model of SCA7.Hum Mol Genet. 2013 Mar 1;22(5):890-903. doi: 10.1093/hmg/dds495. Epub 2012 Nov 29. Hum Mol Genet. 2013. PMID: 23197655 Free PMC article.
-
Molecular Mechanisms and Therapeutic Strategies in Spinocerebellar Ataxia Type 7.Adv Exp Med Biol. 2018;1049:197-218. doi: 10.1007/978-3-319-71779-1_9. Adv Exp Med Biol. 2018. PMID: 29427104 Review.
-
Frequency of SCA1, SCA2, SCA3/MJD, SCA6, SCA7, and DRPLA CAG trinucleotide repeat expansion in patients with hereditary spinocerebellar ataxia from Chinese kindreds.Arch Neurol. 2000 Apr;57(4):540-4. doi: 10.1001/archneur.57.4.540. Arch Neurol. 2000. PMID: 10768629
-
Spinocerebellar ataxia type 7 cerebellar disease requires the coordinated action of mutant ataxin-7 in neurons and glia, and displays non-cell-autonomous bergmann glia degeneration.J Neurosci. 2011 Nov 9;31(45):16269-78. doi: 10.1523/JNEUROSCI.4000-11.2011. J Neurosci. 2011. PMID: 22072678 Free PMC article.
Cited by
-
The Role of Protein Quantity Control in Polyglutamine Spinocerebellar Ataxias.Cerebellum. 2024 Dec;23(6):2575-2592. doi: 10.1007/s12311-024-01722-w. Epub 2024 Jul 25. Cerebellum. 2024. PMID: 39052145 Review.
-
Localization of Potential Energy in Hydrogen Bonds of the ATXN2 Gene.Int J Mol Sci. 2025 Jan 23;26(3):933. doi: 10.3390/ijms26030933. Int J Mol Sci. 2025. PMID: 39940702 Free PMC article.
-
Stability of the CAG Tract in the ATXN2 Gene Depends on the Localization of CAA Interruptions.Biomedicines. 2024 Jul 24;12(8):1648. doi: 10.3390/biomedicines12081648. Biomedicines. 2024. PMID: 39200113 Free PMC article.
-
CAG-targeted brain-permeable therapy tested in biallelic humanized polyQ mouse models.Mol Ther Nucleic Acids. 2025 Feb 22;36(2):102496. doi: 10.1016/j.omtn.2025.102496. eCollection 2025 Jun 10. Mol Ther Nucleic Acids. 2025. PMID: 40171277 Free PMC article.
References
-
- Rub U., Schols L., Paulson H., Auburger G., Kermer P., Jen J.C., Seidel K., Korf H.W., Deller T. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Prog. Neurobiol. 2013;104:38–66. doi: 10.1016/j.pneurobio.2013.01.001. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

