Current Fertility Preservation Steps in Young Women Suffering from Cancer and Future Perspectives
- PMID: 38673945
- PMCID: PMC11050570
- DOI: 10.3390/ijms25084360
Current Fertility Preservation Steps in Young Women Suffering from Cancer and Future Perspectives
Abstract
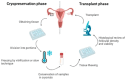
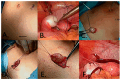
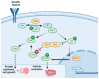
Childhood cancer incidence, especially in high-income countries, has led to a focus on preserving fertility in this vulnerable population. The common treatments, such as radiation and certain chemotherapeutic agents, though effective, pose a risk to fertility. For adult women, established techniques like embryo and egg freezing are standard, requiring ovarian stimulation. However, for prepubescent girls, ovarian tissue freezing has become the primary option, eliminating the need for hormonal preparation. This review describes the beginning, evolution, and current situation of the fertility preservation options for this young population. A total of 75 studies were included, covering the steps in the current fertility preservation protocols: (i) ovarian tissue extraction, (ii) the freezing method, and (iii) thawing and transplantation. Cryopreservation and the subsequent transplantation of ovarian tissue have resulted in successful fertility restoration, with over 200 recorded live births, including cases involving ovarian tissue cryopreserved from prepubescent girls. Despite promising results, challenges persist, such as follicular loss during transplantation, which is attributed to ischemic and oxidative damage. Optimizing ovarian tissue-freezing processes and exploring alternatives to transplantation, like in vitro systems for follicles to establish maturation, are essential to mitigating associated risks. Further research is required in fertility preservation techniques to enhance clinical outcomes in the future. Ovarian tissue cryopreservation appears to be a method with specific benefits, indications, and risks, which can be an important tool in terms of preserving fertility in younger women.
Keywords: cancer treatment; cryopreservation; fertility preservation; follicular loss; ovarian tissue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Pesce R., Marconi M., Vélez C., Marconi G., Glujovsky D., Baronio M., Coscia A. Preservación de la fertilidad. Reproducción. 2017;32:34–39.
-
- World Health Organization [Internet] 2021. [(accessed on 12 December 2022)]. Available online: https://www.who.int/es.
-
- Sheshpari S., Shahnazi M., Mobarak H., Ahmadian S., Bedate A.M., Nariman-Saleh-Fam Z., Nouri M., Rahbarghazi R., Mahdipour M. Ovarian function and reproductive outcome after ovarian tissue transplantation: A systematic review. J. Transl. Med. 2019;17:396. doi: 10.1186/s12967-019-02149-2. - DOI - PMC - PubMed
-
- Batiza Resendiz V.A., Aguilar Melgar A., Luna Rojas R.M., Pérez-Peña E., Gutiérrez Gutiérrez A., Ruvalcaba Castrellón L.A., Salazar López-Ortiz C., Michel Vergara J.A., Shaw Dulin R.J., Barquet Muñoz S.A., et al. Preservación de la fertilidad: Opinión de un grupo de expertos. Ginecol. Obs. Mex. 2020;88:767–805.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

