Low-Protein Diets Differentially Regulate Energy Balance during Thermoneutral and Heat Stress in Cobb Broiler Chicken (Gallus domesticus)
- PMID: 38673954
- PMCID: PMC11050574
- DOI: 10.3390/ijms25084369
Low-Protein Diets Differentially Regulate Energy Balance during Thermoneutral and Heat Stress in Cobb Broiler Chicken (Gallus domesticus)
Abstract
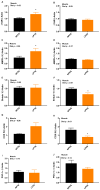
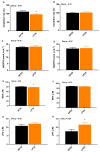
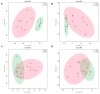
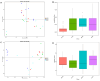
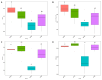
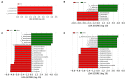
The objective was to assess whether low-protein (LP) diets regulate food intake (FI) and thermogenesis differently during thermoneutral (TN) and heat stress (HS) conditions. Two-hundred-day-old male broiler chicks were weight-matched and assigned to 36 pens with 5-6 chicks/pen. After 2 weeks of acclimation, birds were subjected into four groups (9 pens/group) including (1) a normal-protein diet under TN (ambient temperature), (2) an LP diet under TN, (3) a normal-protein diet under HS (35 °C for 7 h/day), and (4) an LP diet under HS, for 4 weeks. During HS, but not TN, LP tended to decrease FI, which might be associated with a lower mRNA abundance of duodenal ghrelin and higher GIP during HS. The LP group had a higher thermal radiation than NP under TN, but during HS, the LP group had a lower thermal radiation than NP. This was linked with higher a transcript of muscle β1AR and AMPKα1 during TN, but not HS. Further, LP increased the gene expression of COX IV during TN but reduced COX IV and the sirtuin 1 abundance during HS. The dietary protein content differentially impacted plasma metabolome during TN and HS with divergent changes in amino acids such as tyrosine and tryptophan. Compared to NP, LP had increased abundances of p_Tenericutes, c_Mollicutes, c_Mollicutes_RF9, and f_tachnospiraceae under HS. Overall, LP diets may mitigate the negative outcome of heat stress on the survivability of birds by reducing FI and heat production. The differential effect of an LP diet on energy balance during TN and HS is likely regulated by gut and skeletal muscle and alterations in plasma metabolites and cecal microbiota.
Keywords: energy balance; heat stress; low-protein diet; thermoneutral.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens.J Anim Sci. 2018 May 4;96(5):1654-1666. doi: 10.1093/jas/sky092. J Anim Sci. 2018. PMID: 29528406 Free PMC article.
-
Growth performance, duodenal morphology and the caecal microbial population in female broiler chickens fed glycine-fortified low protein diets under heat stress conditions.Br Poult Sci. 2018 Jun;59(3):340-348. doi: 10.1080/00071668.2018.1440377. Epub 2018 Mar 9. Br Poult Sci. 2018. PMID: 29433333
-
Effect of a synbiotic supplement as an antibiotic alternative on broiler skeletal, physiological, and oxidative parameters under heat stress.Poult Sci. 2022 Apr;101(4):101769. doi: 10.1016/j.psj.2022.101769. Epub 2022 Feb 1. Poult Sci. 2022. PMID: 35247651 Free PMC article.
-
A review of heat stress in chickens. Part II: Insights into protein and energy utilization and feeding.Front Physiol. 2022 Aug 8;13:943612. doi: 10.3389/fphys.2022.943612. eCollection 2022. Front Physiol. 2022. PMID: 36003648 Free PMC article. Review.
-
A review of heat stress in chickens. Part I: Insights into physiology and gut health.Front Physiol. 2022 Aug 4;13:934381. doi: 10.3389/fphys.2022.934381. eCollection 2022. Front Physiol. 2022. PMID: 35991182 Free PMC article. Review.
Cited by
-
Transcriptomic analysis of broiler chickens reveals metabolic adaptations to a reduced crude protein diet.Poult Sci. 2025 Apr;104(4):104920. doi: 10.1016/j.psj.2025.104920. Epub 2025 Feb 17. Poult Sci. 2025. PMID: 39983527 Free PMC article.
-
Dietary Insulinogenic Amino Acid Restriction Improves Glucose Metabolism in a Neonatal Piglet Model.Nutrients. 2025 May 15;17(10):1675. doi: 10.3390/nu17101675. Nutrients. 2025. PMID: 40431415 Free PMC article.
-
Residual feed intake in late-laying hens: immune function, metabolic efficiency, and feed utilization dynamics.Front Vet Sci. 2025 Jul 7;12:1624978. doi: 10.3389/fvets.2025.1624978. eCollection 2025. Front Vet Sci. 2025. PMID: 40693113 Free PMC article.
References
-
- Syafwan S., Kwakkel R., Verstegen M. Heat stress and feeding strategies in meat-type chickens. World’s Poult. Sci. J. 2011;67:653–674. doi: 10.1017/S0043933911000742. - DOI
-
- Waldroup P.W. Influence of environmental temperature on protein and amino acid needs of poultry. Fed. Proc. 1982;41:2821–2823. - PubMed
-
- Gous R.M., Morris T.R. Nutritional interventions in alleviating the effects of high temperatures in broiler production. World’s Poult. Sci. J. 2005;61:463–475. doi: 10.1079/WPS200568. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

