Targeted Clindamycin Delivery Systems: Promising Options for Preventing and Treating Bacterial Infections Using Biomaterials
- PMID: 38673971
- PMCID: PMC11050486
- DOI: 10.3390/ijms25084386
Targeted Clindamycin Delivery Systems: Promising Options for Preventing and Treating Bacterial Infections Using Biomaterials
Abstract
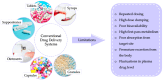
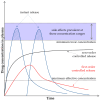
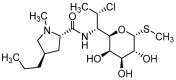
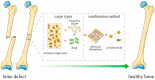
Targeted therapy represents a real opportunity to improve the health and lives of patients. Developments in this field are confirmed by the fact that the global market for drug carriers was worth nearly $40 million in 2022. For this reason, materials engineering and the development of new drug carrier compositions for targeted therapy has become a key area of research in pharmaceutical drug delivery in recent years. Ceramics, polymers, and metals, as well as composites, are of great interest, as when they are appropriately processed or combined with each other, it is possible to obtain biomaterials for hard tissues, soft tissues, and skin applications. After appropriate modification, these materials can release the drug directly at the site requiring a therapeutic effect. This brief literature review characterizes routes of drug delivery into the body and discusses biomaterials from different groups, options for their modification with clindamycin, an antibiotic used for infections caused by aerobic and anaerobic Gram-positive bacteria, and different methods for the final processing of carriers. Examples of coating materials for skin wound healing, acne therapy, and bone tissue fillers are given. Furthermore, the reasons why the use of antibiotic therapy is crucial for a smooth and successful recovery and the risks of bacterial infections are explained. It was demonstrated that there is no single proven delivery scheme, and that the drug can be successfully released from different carriers depending on the destination.
Keywords: antibiotic; biomaterials; clindamycin; drug delivery systems; surgical site infection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ghanem A.M. Recent advances in transdermal drug delivery systems: A review. Int. J. Appl. Pharm. 2024;16:28–33. doi: 10.22159/ijap.2024v16i2.49950. - DOI
-
- Singh S., Pandey V.K., Tewari R.P., Agarwal V. Nanoparticle based drug delivery system: Advantages and applications. Ind. J. Sci. Technol. 2011;4:177–180. doi: 10.17485/ijst/2011/v4i3.16. - DOI
-
- Drug Delivery Systems Market Size, Share & COVID-19 Impact Analysis, by Type (Inhalation, Transdermal, Injectable, and Others), by Device Type (Conventional and Advaced), by Distribution Channel (Hospital, Pharmacies, Retail Pharmacies, and Others) [(accessed on 11 March 2024)]. Available online: https://www.fortunebusinessinsights.com/drug-delivery-systems-market-103070.
-
- Dash T.R., Verma P. Matrix tablets: An approach towards oral extended release drug delivery. Int. J. Pharma Res. Rev. 2013;2:12–24.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

