An Overview of Frog Skin-Derived Esc Peptides: Promising Multifunctional Weapons against Pseudomonas aeruginosa-Induced Pulmonary and Ocular Surface Infections
- PMID: 38673985
- PMCID: PMC11049899
- DOI: 10.3390/ijms25084400
An Overview of Frog Skin-Derived Esc Peptides: Promising Multifunctional Weapons against Pseudomonas aeruginosa-Induced Pulmonary and Ocular Surface Infections
Abstract
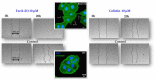
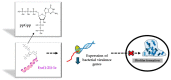
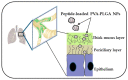
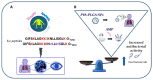
Antimicrobial resistance is a silent pandemic harming human health, and Pseudomonas aeruginosa is the most common bacterium responsible for chronic pulmonary and eye infections. Antimicrobial peptides (AMPs) represent promising alternatives to conventional antibiotics. In this review, the in vitro/in vivo activities of the frog skin-derived AMP Esc(1-21) are shown. Esc(1-21) rapidly kills both the planktonic and sessile forms of P. aeruginosa and stimulates migration of epithelial cells, likely favoring repair of damaged tissue. However, to undertake preclinical studies, some drawbacks of AMPs (cytotoxicity, poor biostability, and limited delivery to the target site) must be overcome. For this purpose, the stereochemistry of two amino acids of Esc(1-21) was changed to obtain the diastereomer Esc(1-21)-1c, which is more stable, less cytotoxic, and more efficient in treating P. aeruginosa-induced lung and cornea infections in mouse models. Incorporation of these peptides (Esc peptides) into nanoparticles or immobilization to a medical device (contact lens) was revealed to be an effective strategy to ameliorate and/or to prolong the peptides' antimicrobial efficacy. Overall, these data make Esc peptides encouraging candidates for novel multifunctional drugs to treat lung pathology especially in patients with cystic fibrosis and eye dysfunctions, characterized by both tissue injury and bacterial infection.
Keywords: D-amino acids; Pseudomonas aeruginosa infections; antimicrobial peptides; cystic fibrosis; delivery systems; frog skin; wound healing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Lepape A., Jean A., De Waele J., Friggeri A., Savey A., Vanhems P., Gustin M.P., Monnet D.L., Garnacho-Montero J., Kohlenberg A. European intensive care physicians’ experience of infections due to antibiotic-resistant bacteria. Antimicrob. Resist. Infect. Control. 2020;9:1. doi: 10.1186/s13756-019-0662-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- PE00000007, INF-ACT/EU funding within the NextGeneration EU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases
- FFC#4/2022 Delegazione FFC Ricerca di Roma e della Franciacorta e Val Camonica/Fondazione Italiana per la Ricerca sulla Fibrosi Cistica
- Anna Tramontano grant 2020/Pasteur-Italia Fondazione Cenci Bolognetti
LinkOut - more resources
Full Text Sources

