The Anticancer Activities of Natural Terpenoids That Inhibit Both Melanoma and Non-Melanoma Skin Cancers
- PMID: 38674007
- PMCID: PMC11050645
- DOI: 10.3390/ijms25084423
The Anticancer Activities of Natural Terpenoids That Inhibit Both Melanoma and Non-Melanoma Skin Cancers
Abstract
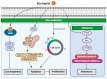
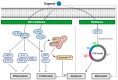
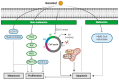
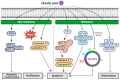
The prevalence of two major types of skin cancer, melanoma and non-melanoma skin cancer, has been increasing worldwide. Skin cancer incidence is estimated to rise continuously over the next 20 years due to ozone depletion and an increased life expectancy. Chemotherapeutic agents could affect healthy cells, and thus may be toxic to them and cause numerous side effects or drug resistance. Phytochemicals that are naturally occurring in fruits, plants, and herbs are known to possess various bioactive properties, including anticancer properties. Although the effects of phytochemicals are relatively milder than chemotherapeutic agents, the long-term intake of phytochemicals may be effective and safe in preventing tumor development in humans. Diverse phytochemicals have shown anti-tumorigenic activities for either melanoma or non-melanoma skin cancer. In this review, we focused on summarizing recent research findings of the natural and dietary terpenoids (eucalyptol, eugenol, geraniol, linalool, and ursolic acid) that have anticancer activities for both melanoma and non-melanoma skin cancers. These terpenoids may be helpful to protect skin collectively to prevent tumorigenesis of both melanoma and nonmelanoma skin cancers.
Keywords: melanoma; natural terpenoids; non-melanoma skin cancer; phytochemicals; skin cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Anticancer effect of terpenes: focus on malignant melanoma.Pharmacol Rep. 2023 Oct;75(5):1115-1125. doi: 10.1007/s43440-023-00512-1. Epub 2023 Jul 29. Pharmacol Rep. 2023. PMID: 37515699 Free PMC article. Review.
-
Phytochemicals for the Management of Melanoma.Mini Rev Med Chem. 2016;16(12):953-79. doi: 10.2174/1389557516666160211120157. Mini Rev Med Chem. 2016. PMID: 26864554 Free PMC article. Review.
-
Nanodelivery Approaches of Phytoactives for Skin Cancers: Current and Future Perspectives.Curr Pharm Biotechnol. 2025;26(5):631-653. doi: 10.2174/0113892010300081240329033208. Curr Pharm Biotechnol. 2025. PMID: 38616742 Review.
-
Heterocyclic Phytochemicals as Anticancer Agents.Curr Top Med Chem. 2025;25(5):533-553. doi: 10.2174/0115680266314693240914070250. Curr Top Med Chem. 2025. PMID: 39350414 Review.
-
Phytochemicals in Anticancer Drug Development.Anticancer Agents Med Chem. 2019;19(2):172-183. doi: 10.2174/1871520618666181106115802. Anticancer Agents Med Chem. 2019. PMID: 30398123 Review.
Cited by
-
Biocompatible Naringin loaded low molecular peptide Nanogels are effective against human melanoma cells.Toxicol Res (Camb). 2024 Nov 7;13(6):tfae185. doi: 10.1093/toxres/tfae185. eCollection 2024 Dec. Toxicol Res (Camb). 2024. PMID: 39524611
-
Preparation of Composite Hydrogels Based on Cysteine-Silver Sol and Methylene Blue as Promising Systems for Anticancer Photodynamic Therapy.Gels. 2024 Sep 5;10(9):577. doi: 10.3390/gels10090577. Gels. 2024. PMID: 39330179 Free PMC article.
-
Gossypin-Loaded Ethosome Gel for Cutaneous Administration: A Preliminary Study on Melanoma Cells.Antioxidants (Basel). 2025 Feb 5;14(2):186. doi: 10.3390/antiox14020186. Antioxidants (Basel). 2025. PMID: 40002373 Free PMC article.
-
Alchemy in Nature: The Role of Lawsonia inermis Extract Choice in Crafting Potent Anticancer Metal Nanoparticles.ACS Appl Mater Interfaces. 2025 Jan 22;17(3):4637-4661. doi: 10.1021/acsami.4c19585. Epub 2025 Jan 11. ACS Appl Mater Interfaces. 2025. PMID: 39798120 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

