Regulation of Cysteine Homeostasis and Its Effect on Escherichia coli Sensitivity to Ciprofloxacin in LB Medium
- PMID: 38674008
- PMCID: PMC11050555
- DOI: 10.3390/ijms25084424
Regulation of Cysteine Homeostasis and Its Effect on Escherichia coli Sensitivity to Ciprofloxacin in LB Medium
Abstract
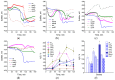
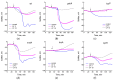
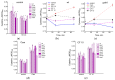
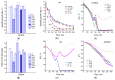
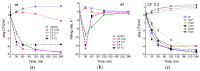
Cysteine and its derivatives, including H2S, can influence bacterial virulence and sensitivity to antibiotics. In minimal sulfate media, H2S is generated under stress to prevent excess cysteine and, together with incorporation into glutathione and export into the medium, is a mechanism of cysteine homeostasis. Here, we studied the features of cysteine homeostasis in LB medium, where the main source of sulfur is cystine, whose import can create excess cysteine inside cells. We used mutants in the mechanisms of cysteine homeostasis and a set of microbiological and biochemical methods, including the real-time monitoring of sulfide and oxygen, the determination of cysteine and glutathione (GSH), and the expression of the Fur, OxyR, and SOS regulons genes. During normal growth, the parental strain generated H2S when switching respiration to another substrate. The mutations affected the onset time, the intensity and duration of H2S production, cysteine and glutathione levels, bacterial growth and respiration rates, and the induction of defense systems. Exposure to chloramphenicol and high doses of ciprofloxacin increased cysteine content and GSH synthesis. A high inverse relationship between log CFU/mL and bacterial growth rate before ciprofloxacin addition was revealed. The study points to the important role of maintaining cysteine homeostasis during normal growth and antibiotic exposure in LB medium.
Keywords: Fur and SOS regulons; H2S; antibiotics; bacterial survival; cysteine homeostasis; glutathione; growth; respiration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Effect of H2S and cysteine homeostasis disturbance on ciprofloxacin sensitivity of Escherichia coli in cystine-free and cystine-fed minimal medium.Arch Microbiol. 2024 Nov 4;206(12):456. doi: 10.1007/s00203-024-04185-z. Arch Microbiol. 2024. PMID: 39495300
-
Cysteine Homeostasis Disturbance in Escherichia coli Caused by Exposure to Ciprofloxacin.Bull Exp Biol Med. 2024 Apr;176(6):791-795. doi: 10.1007/s10517-024-06110-2. Epub 2024 Jun 19. Bull Exp Biol Med. 2024. PMID: 38890214
-
Cysteine homeostasis under inhibition of protein synthesis in Escherichia coli cells.Amino Acids. 2019 Nov;51(10-12):1577-1592. doi: 10.1007/s00726-019-02795-2. Epub 2019 Oct 15. Amino Acids. 2019. PMID: 31617110
-
Cysteine Metabolism in Tumor Redox Homeostasis.Curr Med Chem. 2023;30(16):1813-1823. doi: 10.2174/0929867329666220817141227. Curr Med Chem. 2023. PMID: 35980069 Review.
-
Hydrogen sulfide in plants: from dissipation of excess sulfur to signaling molecule.Nitric Oxide. 2014 Sep 15;41:72-8. doi: 10.1016/j.niox.2014.02.005. Epub 2014 Feb 26. Nitric Oxide. 2014. PMID: 24582856 Review.
Cited by
-
Identification of Fungal Metabolite Gliotoxin as a Potent Inhibitor Against Bacterial O-Acetylserine Sulfhydrylase CysK and CysM.Int J Mol Sci. 2025 Jan 27;26(3):1106. doi: 10.3390/ijms26031106. Int J Mol Sci. 2025. PMID: 39940875 Free PMC article.
-
Effect of H2S and cysteine homeostasis disturbance on ciprofloxacin sensitivity of Escherichia coli in cystine-free and cystine-fed minimal medium.Arch Microbiol. 2024 Nov 4;206(12):456. doi: 10.1007/s00203-024-04185-z. Arch Microbiol. 2024. PMID: 39495300
-
Stress-Induced Sulfide Production by Bacillus subtilis and Bacillus megaterium.Microorganisms. 2024 Sep 7;12(9):1856. doi: 10.3390/microorganisms12091856. Microorganisms. 2024. PMID: 39338531 Free PMC article.
-
Ammonium depletion induces changes in low molecular weight thiol levels in Escherichia coli and Bacillus subtilis.Arch Microbiol. 2025 Aug 28;207(10):238. doi: 10.1007/s00203-025-04445-6. Arch Microbiol. 2025. PMID: 40875049
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

