Elimination of Pathogen Biofilms via Postbiotics from Lactic Acid Bacteria: A Promising Method in Food and Biomedicine
- PMID: 38674648
- PMCID: PMC11051744
- DOI: 10.3390/microorganisms12040704
Elimination of Pathogen Biofilms via Postbiotics from Lactic Acid Bacteria: A Promising Method in Food and Biomedicine
Abstract
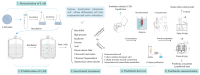
Pathogenic biofilms provide a naturally favorable barrier for microbial growth and are closely related to the virulence of pathogens. Postbiotics from lactic acid bacteria (LAB) are secondary metabolites and cellular components obtained by inactivation of fermentation broth; they have a certain inhibitory effect on all stages of pathogen biofilms. Postbiotics from LAB have drawn attention because of their high stability, safety dose parameters, and long storage period, which give them a broad application prospect in the fields of food and medicine. The mechanisms of eliminating pathogen biofilms via postbiotics from LAB mainly affect the surface adhesion, self-aggregation, virulence, and QS of pathogens influencing interspecific and intraspecific communication. However, there are some factors (preparation process and lack of target) which can limit the antibiofilm impact of postbiotics. Therefore, by using a delivery carrier and optimizing process parameters, the effect of interfering factors can be eliminated. This review summarizes the concept and characteristics of postbiotics from LAB, focusing on their preparation technology and antibiofilm effect, and the applications and limitations of postbiotics in food processing and clinical treatment are also discussed.
Keywords: antibiofilm agents; bioactive substances; biomedical function; food safety; postbiotics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Vunduk J., Wan-Mohtar W.A.A.Q.I., Mohamad S.A., Abd Halim N.H., Mohd Dzomir A.Z., Žižak Ž., Klaus A. Polysaccharides of Pleurotus flabellatus strain Mynuk produced by submerged fermentation as a promising novel tool against adhesion and biofilm formation of foodborne pathogens. Lwt. 2019;112:108221. doi: 10.1016/j.lwt.2019.05.119. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

