Ligand-Free Silver Nanoparticles: An Innovative Strategy against Viruses and Bacteria
- PMID: 38674764
- PMCID: PMC11052337
- DOI: 10.3390/microorganisms12040820
Ligand-Free Silver Nanoparticles: An Innovative Strategy against Viruses and Bacteria
Abstract
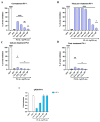
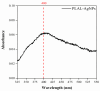
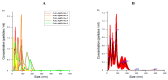
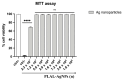
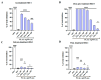
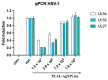
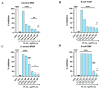
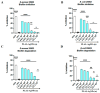
The spread of antibiotic-resistant bacteria and the rise of emerging and re-emerging viruses in recent years constitute significant public health problems. Therefore, it is necessary to develop new antimicrobial strategies to overcome these challenges. Herein, we describe an innovative method to synthesize ligand-free silver nanoparticles by Pulsed Laser Ablation in Liquid (PLAL-AgNPs). Thus produced, nanoparticles were characterized by total X-ray fluorescence, zeta potential analysis, transmission electron microscopy (TEM), and nanoparticle tracking analysis (NTA). A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to evaluate the nanoparticles' cytotoxicity. Their potential was evaluated against the enveloped herpes simplex virus type 1 (HSV-1) and the naked poliovirus type 1 (PV-1) by plaque reduction assays and confirmed by real-time PCR and fluorescence microscopy, showing that nanoparticles interfered with the early stage of infection. Their action was also examined against different bacteria. We observed that the PLAL-AgNPs exerted a strong effect against both methicillin-resistant Staphylococcus aureus (S. aureus MRSA) and Escherichia coli (E. coli) producing extended-spectrum β-lactamase (ESBL). In detail, the PLAL-AgNPs exhibited a bacteriostatic action against S. aureus and a bactericidal activity against E. coli. Finally, we proved that the PLAL-AgNPs were able to inhibit/degrade the biofilm of S. aureus and E. coli.
Keywords: PLAL-AgNPs; antibacterial activity; antibiofilm activity; antiviral activity; herpesvirus; multidrug resistance; poliovirus; silver nanoparticles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Silver Nanoparticle-Mediated Antiviral Efficacy against Enveloped Viruses: A Comprehensive Review.Glob Chall. 2025 Mar 28;9(5):2400380. doi: 10.1002/gch2.202400380. eCollection 2025 May. Glob Chall. 2025. PMID: 40352632 Free PMC article. Review.
-
Biosynthesis of Silver Nanoparticles Using the Biofilm Supernatant of Pseudomonas aeruginosa PA75 and Evaluation of Their Antibacterial, Antibiofilm, and Antitumor Activities.Int J Nanomedicine. 2023 May 10;18:2485-2502. doi: 10.2147/IJN.S410314. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37192897 Free PMC article.
-
Toxicity and antibacterial assessment of chitosan-coated silver nanoparticles on human pathogens and macrophage cells.Int J Nanomedicine. 2012;7:1805-18. doi: 10.2147/IJN.S28077. Epub 2012 Apr 3. Int J Nanomedicine. 2012. PMID: 22619529 Free PMC article.
-
Green Synthesized Silver Nanoparticles: Antibacterial and Anticancer Activities, Biocompatibility, and Analyses of Surface-Attached Proteins.Front Microbiol. 2021 Apr 22;12:632505. doi: 10.3389/fmicb.2021.632505. eCollection 2021. Front Microbiol. 2021. PMID: 33967977 Free PMC article.
-
Antibacterial and antibiofilm potential of silver nanoparticles against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains.Braz J Microbiol. 2021 Mar;52(1):267-278. doi: 10.1007/s42770-020-00406-x. Epub 2020 Nov 24. Braz J Microbiol. 2021. PMID: 33231865 Free PMC article. Review.
Cited by
-
Silver Nanoparticle-Mediated Antiviral Efficacy against Enveloped Viruses: A Comprehensive Review.Glob Chall. 2025 Mar 28;9(5):2400380. doi: 10.1002/gch2.202400380. eCollection 2025 May. Glob Chall. 2025. PMID: 40352632 Free PMC article. Review.
References
-
- Van Seventer J.M., Hochberg N.S. International Encyclopedia of Public Health. Elsevier; Amsterdam, The Netherlands: 2017. Principles of Infectious Diseases: Transmission, Diagnosis, Prevention, and Control; pp. 22–39.
-
- Ismahene Y. Infectious Diseases, Trade, and Economic Growth: A Panel Analysis of Developed and Developing Countries. J. Knowl. Econ. 2022;13:2547–2583. doi: 10.1007/s13132-021-00811-z. - DOI
-
- Anton-Vazquez V., Mehra V., Mbisa J.L., Bradshaw D., Basu T.N., Daly M.-L., Mufti G.J., Pagliuca A., Potter V., Zuckerman M. Challenges of aciclovir-resistant HSV infection in allogeneic bone marrow transplant recipients. J. Clin. Virol. 2020;128:104421. doi: 10.1016/j.jcv.2020.104421. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

