Faecalibacterium prausnitzii Supplementation Prevents Intestinal Barrier Injury and Gut Microflora Dysbiosis Induced by Sleep Deprivation
- PMID: 38674791
- PMCID: PMC11054126
- DOI: 10.3390/nu16081100
Faecalibacterium prausnitzii Supplementation Prevents Intestinal Barrier Injury and Gut Microflora Dysbiosis Induced by Sleep Deprivation
Abstract
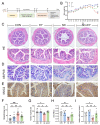
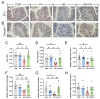
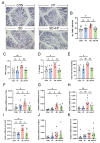
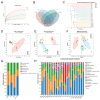
Sleep deprivation (SD) leads to impaired intestinal barrier function and intestinal flora disorder, especially a reduction in the abundance of the next generation of probiotic Faecalibacterium prausnitzii (F. prausnitzii). However, it remains largely unclear whether F. prausnitzii can ameliorate SD-induced intestinal barrier damage. A 72 h SD mouse model was used in this research, with or without the addition of F. prausnitzii. The findings indicated that pre-colonization with F. prausnitzii could protect against tissue damage from SD, enhance goblet cell count and MUC2 levels in the colon, boost tight-junction protein expression, decrease macrophage infiltration, suppress pro-inflammatory cytokine expression, and reduce apoptosis. We found that the presence of F. prausnitzii helped to balance the gut microbiota in SD mice by reducing harmful bacteria like Klebsiella and Staphylococcus, while increasing beneficial bacteria such as Akkermansia. Ion chromatography analysis revealed that F. prausnitzii pretreatment increased the fecal butyrate level in SD mice. Overall, these results suggested that incorporating F. prausnitzii could help reduce gut damage caused by SD, potentially by enhancing the intestinal barrier and balancing gut microflora. This provides a foundation for utilizing probiotics to protect against intestinal illnesses.
Keywords: Faecalibacterium prausnitzii; intestinal barrier function; intestinal microflora; short chain fatty acids; sleep deprivation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

