Changes in the Progression of Chronic Kidney Disease in Patients Undergoing Fecal Microbiota Transplantation
- PMID: 38674803
- PMCID: PMC11055146
- DOI: 10.3390/nu16081109
Changes in the Progression of Chronic Kidney Disease in Patients Undergoing Fecal Microbiota Transplantation
Abstract
Chronic kidney disease (CKD) is a progressive loss of renal function in which gut dysbiosis is involved. Fecal microbiota transplantation (FMT) may be a promising alternative for restoring gut microbiota and treating CKD. This study evaluated the changes in CKD progression in patients treated with FMT. Patients with diabetes and/or hypertension with CKD clinical stages 2, 3, and 4 in this single-center, double-blind, randomized, placebo-controlled clinical trial (NCT04361097) were randomly assigned to receive either FMT or placebo capsules for 6 months. Laboratory and stool metagenomic analyses were performed. A total of 28 patients were included (15 FMT and 13 placebo). Regardless of CKD stages, patients responded similarly to FMT treatment. More patients (53.8%) from the placebo group progressed to CKD than the FMT group (13.3%). The FMT group maintained stable renal function parameters (serum creatinine and urea nitrogen) compared to the placebo group. Adverse events after FMT treatment were mild or moderate gastrointestinal symptoms. The abundance of Firmicutes and Actinobacteria decreased whereas Bacteroidetes, Proteobacteria and Roseburia spp. increased in the FMT group. CKD patients showed less disease progression after FMT administration. The administration of oral FMT in patients with CKD is a safe strategy, does not represent a risk, and has potential benefits.
Keywords: chronic kidney disease; disease progression; fecal microbiota transplant.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
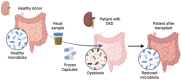
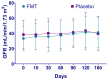
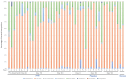
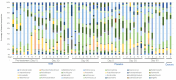
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

