Novel Fermentates Can Enhance Key Immune Responses Associated with Viral Immunity
- PMID: 38674902
- PMCID: PMC11053696
- DOI: 10.3390/nu16081212
Novel Fermentates Can Enhance Key Immune Responses Associated with Viral Immunity
Abstract
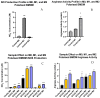
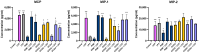
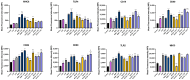
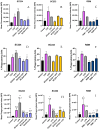
Fermented foods have long been known to have immunomodulatory capabilities, and fermentates derived from the lactic acid bacteria of dairy products can modulate the immune system. We have used skimmed milk powder to generate novel fermentates using Lb. helveticus strains SC234 and SC232 and we demonstrate here that these fermentates can enhance key immune mechanisms that are critical to the immune response to viruses. We show that our novel fermentates, SC234 and SC232, can positively impact on cytokine and chemokine secretion, nitric oxide (NO) production, cell surface marker expression, and phagocytosis in macrophage models. We demonstrate that the fermentates SC234 and SC232 increase the secretion of cytokines IL-1β, IL-6, TNF-α, IL-27, and IL-10; promote an M1 pro-inflammatory phenotype for viral immunity via NO induction; decrease chemokine expression of Monocyte Chemoattractant Protein (MCP); increase cell surface marker expression; and enhance phagocytosis in comparison to their starting material. These data suggest that these novel fermentates have potential as novel functional food ingredients for the treatment, management, and control of viral infection.
Keywords: fermentates; functional food; immune boosting; immunomodulation; macrophage; viral immunity.
Conflict of interest statement
Authors Monica A. Mechoud, Tom Beresford, Harsh Mathur, Paul D. Cotter were employed by the company Teagasc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- García-Burgos M., Moreno-Fernández J., Alférez M.J., Díaz-Castro J., López-Aliaga I. New Perspectives in Fermented Dairy Products and Their Health Relevance. J. Funct. Foods. 2020;72:104059. doi: 10.1016/j.jff.2020.104059. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

