Biopolymeric Nanocomposites for CO2 Capture
- PMID: 38674984
- PMCID: PMC11054771
- DOI: 10.3390/polym16081063
Biopolymeric Nanocomposites for CO2 Capture
Abstract
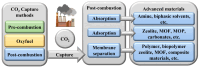
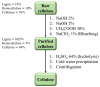
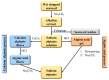
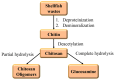
Carbon dioxide (CO2) impacts the greenhouse effect significantly and results in global warming, prompting urgent attention to climate change concerns. In response, CO2 capture has emerged as a crucial process to capture carbon produced in industrial and power processes before its release into the atmosphere. The main aim of CO2 capture is to mitigate the emissions of greenhouse gas and reduce the anthropogenic impact on climate change. Biopolymer nanocomposites offer a promising avenue for CO2 capture due to their renewable nature. These composites consist of biopolymers derived from biological sources and nanofillers like nanoparticles and nanotubes, enhancing the properties of the composite. Various biopolymers like chitosan, cellulose, carrageenan, and others, possessing unique functional groups, can interact with CO2 molecules. Nanofillers are incorporated to improve mechanical, thermal, and sorption properties, with materials such as graphene, carbon nanotubes, and metallic nanoparticles enhancing surface area and porosity. The CO2 capture mechanism within biopolymer nanocomposites involves physical absorption, chemisorption, and physisorption, driven by functional groups like amino and hydroxyl groups in the biopolymer matrix. The integration of nanofillers further boosts CO2 adsorption capacity by increasing surface area and porosity. Numerous advanced materials, including biopolymeric derivatives like cellulose, alginate, and chitosan, are developed for CO2 capture technology, offering accessibility and cost-effectiveness. This semi-systematic literature review focuses on recent studies involving biopolymer-based materials for CO2 capture, providing an overview of composite materials enriched with nanomaterials, specifically based on cellulose, alginate, chitosan, and carrageenan; the choice of these biopolymers is dictated by the lack of a literature perspective focused on a currently relevant topic such as these biorenewable resources in the framework of carbon capture. The production and efficacy of biopolymer-based adsorbents and membranes are examined, shedding light on potential trends in global CO2 capture technology enhancement.
Keywords: CO2 capture; adsorption capacity; biopolymer; carbon dioxide; nanocomposite materials.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Recent advancements in engineered biopolymeric-nanohybrids: A greener approach for adsorptive-remediation of noxious metals from aqueous matrices.Environ Res. 2022 Dec;215(Pt 3):114398. doi: 10.1016/j.envres.2022.114398. Epub 2022 Sep 26. Environ Res. 2022. PMID: 36174757 Review.
-
Kinetics and selectivity insights into carbon dioxide capture utilizing carboxymethyl cellulose-polypyrrole nanocomposites: Screening of silane functionalization.Carbohydr Polym. 2025 May 15;356:123399. doi: 10.1016/j.carbpol.2025.123399. Epub 2025 Feb 16. Carbohydr Polym. 2025. PMID: 40049970
-
Enhanced CO2 Capture Potential of Chitosan-Based Composite Beads by Adding Activated Carbon from Coffee Grounds and Crosslinking with Epichlorohydrin.Int J Mol Sci. 2024 Aug 16;25(16):8916. doi: 10.3390/ijms25168916. Int J Mol Sci. 2024. PMID: 39201602 Free PMC article.
-
Critical review of existing nanomaterial adsorbents to capture carbon dioxide and methane.Sci Total Environ. 2017 Oct 1;595:51-62. doi: 10.1016/j.scitotenv.2017.03.229. Epub 2017 Apr 1. Sci Total Environ. 2017. PMID: 28376428 Review.
-
Research on carbon dioxide capture materials used for carbon dioxide capture, utilization, and storage technology: a review.Environ Sci Pollut Res Int. 2024 May;31(23):33259-33302. doi: 10.1007/s11356-024-33370-2. Epub 2024 May 3. Environ Sci Pollut Res Int. 2024. PMID: 38698095 Review.
Cited by
-
CO2 Capture: A Comprehensive Review and Bibliometric Analysis of Scalable Materials and Sustainable Solutions.Molecules. 2025 Jan 26;30(3):563. doi: 10.3390/molecules30030563. Molecules. 2025. PMID: 39942667 Free PMC article. Review.
-
Tailored Carbon Nanocomposites for Efficient CO2 Capture.Molecules. 2025 May 30;30(11):2408. doi: 10.3390/molecules30112408. Molecules. 2025. PMID: 40509295 Free PMC article.
-
Polymeric Membrane Contactors for CO2 Separation: A Systematic Literature Analysis of the Impact of Absorbent Temperature.Polymers (Basel). 2025 May 18;17(10):1387. doi: 10.3390/polym17101387. Polymers (Basel). 2025. PMID: 40430682 Free PMC article.
References
-
- Raza S., Orooji Y., Ghasali E., Hayat A., Karimi-Maleh H., Lin H. Engineering Approaches for CO2 Converting to Biomass Coupled with Nanobiomaterials as Biomediated towards Circular Bioeconomy. J. CO2 Util. 2023;67:102295. doi: 10.1016/j.jcou.2022.102295. - DOI
-
- Cheung O., Hedin N. Zeolites and Related Sorbents with Narrow Pores for CO2 Separation from Flue Gas. RSC Adv. 2014;4:14480–14494. doi: 10.1039/C3RA48052F. - DOI
-
- Villari V., Mazzaglia A., Trapani M., Castriciano M.A., de Luca G., Romeo A., Scolaro L.M., Micali N. Optical Enhancement and Structural Properties of a Hybrid Organic−Inorganic Ternary Nanocomposite. J. Phys. Chem. C. 2011;115:5435–5439. doi: 10.1021/jp110966h. - DOI
-
- Li Y., Jia P., Xu J., Wu Y., Jiang H., Li Z. The Aminosilane Functionalization of Cellulose Nanofibrils and the Mechanical and CO2 Adsorption Characteristics of Their Aerogel. Ind. Eng. Chem. Res. 2020;59:2874–2882. doi: 10.1021/acs.iecr.9b04253. - DOI
Publication types
LinkOut - more resources
Full Text Sources

