Glucose Metabolism as a Potential Therapeutic Target in Cytarabine-Resistant Acute Myeloid Leukemia
- PMID: 38675105
- PMCID: PMC11055074
- DOI: 10.3390/pharmaceutics16040442
Glucose Metabolism as a Potential Therapeutic Target in Cytarabine-Resistant Acute Myeloid Leukemia
Abstract
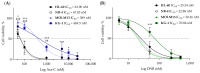
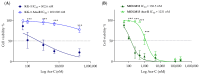
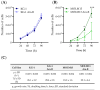
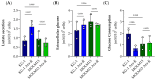
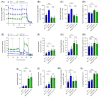
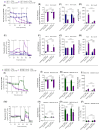
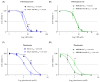
Altered glycolytic metabolism has been associated with chemoresistance in acute myeloid leukemia (AML). However, there are still aspects that need clarification, as well as how to explore these metabolic alterations in therapy. In the present study, we aimed to elucidate the role of glucose metabolism in the acquired resistance of AML cells to cytarabine (Ara-C) and to explore it as a therapeutic target. Resistance was induced by stepwise exposure of AML cells to increasing concentrations of Ara-C. Ara-C-resistant cells were characterized for their growth capacity, genetic alterations, metabolic profile, and sensitivity to different metabolic inhibitors. Ara-C-resistant AML cell lines, KG-1 Ara-R, and MOLM13 Ara-R presented different metabolic profiles. KG-1 Ara-R cells exhibited a more pronounced glycolytic phenotype than parental cells, with a weaker acute response to 3-bromopyruvate (3-BP) but higher sensitivity after 48 h. KG-1 Ara-R cells also display increased respiration rates and are more sensitive to phenformin than parental cells. On the other hand, MOLM13 Ara-R cells display a glucose metabolism profile similar to parental cells, as well as sensitivity to glycolytic inhibitors. These results indicate that acquired resistance to Ara-C in AML may involve metabolic adaptations, which can be explored therapeutically in the AML patient setting who developed resistance to therapy.
Keywords: 3-bromopyruvate; acute myeloid leukemia; chemoresistance; cytarabine; glucose metabolism; metabolic inhibitors; phenformin; seahorse.
Conflict of interest statement
Y.H.K. was co-founder of the company NewG Lab Pharma and KoDiscovery. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Disulfiram overcomes bortezomib and cytarabine resistance in Down-syndrome-associated acute myeloid leukemia cells.J Exp Clin Cancer Res. 2017 Feb 1;36(1):22. doi: 10.1186/s13046-017-0493-5. J Exp Clin Cancer Res. 2017. PMID: 28143565 Free PMC article.
-
Trp53 loss during in vitro selection contributes to acquired Ara-C resistance in acute myeloid leukemia.Exp Hematol. 2006 May;34(5):631-41. doi: 10.1016/j.exphem.2006.01.015. Exp Hematol. 2006. PMID: 16647569
-
Defective expression of deoxycytidine kinase in cytarabine-resistant acute myeloid leukemia cells.Int J Oncol. 2009 Apr;34(4):1165-71. doi: 10.3892/ijo_00000245. Int J Oncol. 2009. PMID: 19287976
-
Inhibitors of Chemoresistance Pathways in Combination with Ara-C to Overcome Multidrug Resistance in AML. A Mini Review.Int J Mol Sci. 2021 May 7;22(9):4955. doi: 10.3390/ijms22094955. Int J Mol Sci. 2021. PMID: 34066940 Free PMC article. Review.
-
Problems related to resistance to cytarabine in acute myeloid leukemia.Leuk Lymphoma. 2004 Jun;45(6):1123-32. doi: 10.1080/1042819032000159861. Leuk Lymphoma. 2004. PMID: 15359991 Review.
Cited by
-
Signal Mining and Analysis of Drug-Induced Myelosuppression: A Real-World Study From FAERS.Cancer Control. 2025 Jan-Dec;32:10732748251337362. doi: 10.1177/10732748251337362. Epub 2025 May 29. Cancer Control. 2025. PMID: 40439714 Free PMC article.
-
Acute Myeloid Leukemia in Older Patients: From New Biological Insights to Targeted Therapies.Curr Oncol. 2024 Oct 24;31(11):6632-6658. doi: 10.3390/curroncol31110490. Curr Oncol. 2024. PMID: 39590121 Free PMC article. Review.
References
-
- Khoury J.D., Solary E., Abla O., Alaggio R., Apperley J.F., Bejar R., Hochhaus A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36:1703–1719. doi: 10.1038/s41375-022-01613-1. - DOI - PMC - PubMed
-
- Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. - DOI - PubMed
-
- Ganzel C., Sun Z., Cripe L.D., Fernandez H.F., Douer D., Rowe J.M., Paietta E.M., Ketterling R., O’Connell M.J., Wiernik P.H., et al. Very poor long-term survival in past and more recent studies for relapsed AML patients: The ECOG-ACRIN experience. Am. J. Hematol. 2018;93:1074–1081. doi: 10.1002/ajh.25162. - DOI - PMC - PubMed
-
- Cancer.Net. Leukemia-Acute Myeloid-AML. [(accessed on 5 August 2023)]. Available online: https://www.cancer.net/cancer-types/leukemia-acute-myeloid-aml/statistic....
-
- Döhner H., Estey E., Grimwade D., Amadori S., Appelbaum F.R., Büchner T., Dombret H., Ebert B.L., Fenaux P., Larson R.A., et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. - DOI - PMC - PubMed
Grants and funding
- SFRH/BD/146065/2019, SFRH/BD/145955/2019, COVID/BD/153456/2023, UIDB/50026/2020 and UIDP/50026/2020/Fundação para a Ciência e Tecnologia
- NORTE-01-0145-FEDER-000039, NORTE-01-0145-FEDER-000055/Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF)
- not applicable/NewG Lab Pharma
- not applicable/Children Cancer Foundation Salzburg, Austria
LinkOut - more resources
Full Text Sources

