Intranasal Administration of Mesenchymal Stem Cell-Derived Exosome Alleviates Hypoxic-Ischemic Brain Injury
- PMID: 38675108
- PMCID: PMC11053690
- DOI: 10.3390/pharmaceutics16040446
Intranasal Administration of Mesenchymal Stem Cell-Derived Exosome Alleviates Hypoxic-Ischemic Brain Injury
Abstract
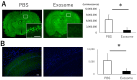
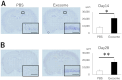
Hypoxic-ischemic brain injury arises from inadequate oxygen delivery to the brain, commonly occurring following cardiac arrest, which lacks effective treatments. Recent studies have demonstrated the therapeutic potential of exosomes released from mesenchymal stem cells. Given the challenge of systemic dilution associated with intravenous administration, intranasal delivery has emerged as a promising approach. In this study, we investigate the effects of intranasally administered exosomes in an animal model. Exosomes were isolated from the cell supernatants using the ultracentrifugation method. Brain injury was induced in Sprague-Dawley rats through a transient four-vessel occlusion model. Intranasal administration was conducted with 3 × 108 exosome particles in 20 µL of PBS or PBS alone, administered daily for 7 days post-injury. Long-term cognitive behavioral assessments, biodistribution of exosomes, and histological evaluations of apoptosis and neuroinflammation were conducted. Exosomes were primarily detected in the olfactory bulb one hour after intranasal administration, subsequently distributing to the striatum and midbrain. Rats treated with exosomes exhibited substantial improvement in cognitive function up to 28 days after the insult, and demonstrated significantly fewer apoptotic cells along with higher neuronal cell survival in the hippocampus. Exosomes were found to be taken up by microglia, leading to a decrease in the expression of cytotoxic inflammatory markers.
Keywords: exosome; hypoxic-ischemic brain injury; inflammation; intranasal administration; mesenchymal stem cell.
Conflict of interest statement
Author, Sho Yamaguchi is employed by the company Kaneka. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Geocadin R.G., Wijdicks E., Armstrong M.J., Damian M., Mayer S.A., Ornato J.P., Rabinstein A., Suarez J.I., Torbey M.T., Dubinsky R.M., et al. Practice Guideline Summary: Reducing Brain Injury Following Cardiopulmonary Resuscitation: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017;88:2141–2149. doi: 10.1212/WNL.0000000000003966. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

