An Application of a Physiologically Based Pharmacokinetic Approach to Predict Ceftazidime Pharmacokinetics in a Pregnant Population
- PMID: 38675135
- PMCID: PMC11054561
- DOI: 10.3390/pharmaceutics16040474
An Application of a Physiologically Based Pharmacokinetic Approach to Predict Ceftazidime Pharmacokinetics in a Pregnant Population
Abstract
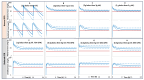
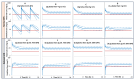
Physiological changes during pregnancy can alter maternal and fetal drug exposure. The objective of this work was to predict maternal and umbilical ceftazidime pharmacokinetics during pregnancy. Ceftazidime transplacental permeability was predicted from its physicochemical properties and incorporated into the model. Predicted concentrations and parameters from the PBPK model were compared to the observed data. PBPK predicted ceftazidime concentrations in non-pregnant and pregnant subjects of different gestational weeks were within 2-fold of the observations, and the observed concentrations fell within the 5th-95th prediction interval from the PBPK simulations. The calculated transplacental clearance (0.00137 L/h/mL of placenta volume) predicted an average umbilical cord-to-maternal plasma ratio of 0.7 after the first dose, increasing to about 1.0 at a steady state, which also agrees well with clinical observations. The developed maternal PBPK model adequately predicted the observed exposure and kinetics of ceftazidime in the pregnant population. Using a verified population-based PBPK model provides valuable insights into the disposition of drug concentrations in special individuals that are otherwise difficult to study and, in addition, offers the possibility of supplementing sparse samples obtained in vulnerable populations with additional knowledge, informing the dosing adjustment and study design, and improving the efficacy and safety of drugs in target populations.
Keywords: GFR; PBPK model; ceftazidime; feto-placenta; pregnancy; renal.
Conflict of interest statement
All authors are paid employees of Certara UK Limited (Certara Predictive Technologies Division) and may hold shares in Certara. The authors indicate no other conflicts of interest.
Figures





Similar articles
-
Application of Physiologically Based Pharmacokinetic Model to Delineate the Impact of Aging and Renal Impairment on Ceftazidime Clearance.Antibiotics (Basel). 2024 Sep 9;13(9):862. doi: 10.3390/antibiotics13090862. Antibiotics (Basel). 2024. PMID: 39335035 Free PMC article.
-
Application of a Physiologically Based Pharmacokinetic Approach to Predict Theophylline Pharmacokinetics Using Virtual Non-Pregnant, Pregnant, Fetal, Breast-Feeding, and Neonatal Populations.Front Pediatr. 2022 May 12;10:840710. doi: 10.3389/fped.2022.840710. eCollection 2022. Front Pediatr. 2022. PMID: 35652056 Free PMC article.
-
Development of a Novel Maternal-Fetal Physiologically Based Pharmacokinetic Model II: Verification of the model for passive placental permeability drugs.Drug Metab Dispos. 2017 Aug;45(8):939-946. doi: 10.1124/dmd.116.073957. Epub 2017 Jan 3. Drug Metab Dispos. 2017. PMID: 28049636 Free PMC article.
-
Predicting Human Fetal Drug Exposure Through Maternal-Fetal PBPK Modeling and In Vitro or Ex Vivo Studies.J Clin Pharmacol. 2022 Sep;62 Suppl 1(Suppl 1):S94-S114. doi: 10.1002/jcph.2117. J Clin Pharmacol. 2022. PMID: 36106781 Free PMC article. Review.
-
A review of pregnancy-induced changes in opioid pharmacokinetics, placental transfer, and fetal exposure: Towards fetomaternal physiologically-based pharmacokinetic modeling to improve the treatment of neonatal opioid withdrawal syndrome.Pharmacol Ther. 2022 Jun;234:108045. doi: 10.1016/j.pharmthera.2021.108045. Epub 2021 Nov 20. Pharmacol Ther. 2022. PMID: 34813863 Review.
Cited by
-
Application of Physiologically Based Pharmacokinetic Model to Delineate the Impact of Aging and Renal Impairment on Ceftazidime Clearance.Antibiotics (Basel). 2024 Sep 9;13(9):862. doi: 10.3390/antibiotics13090862. Antibiotics (Basel). 2024. PMID: 39335035 Free PMC article.
-
Medicines in pregnancy: A clinical pharmacology extrapolation framework to address knowledge gaps.CPT Pharmacometrics Syst Pharmacol. 2024 Nov;13(11):1830-1834. doi: 10.1002/psp4.13242. Epub 2024 Oct 14. CPT Pharmacometrics Syst Pharmacol. 2024. PMID: 39400534 Free PMC article. No abstract available.
-
PBPK Modeling of Lamotrigine and Efavirenz during Pregnancy: Implications for Personalized Dosing and Drug-Drug Interaction Management.Pharmaceutics. 2024 Sep 3;16(9):1163. doi: 10.3390/pharmaceutics16091163. Pharmaceutics. 2024. PMID: 39339201 Free PMC article.
References
-
- Abduljalil K., Furness P., Johnson T.N., Rostami-Hodjegan A., Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: A database for parameters required in physiologically based pharmacokinetic modelling. Clin. Pharmacokinet. 2012;51:365–396. doi: 10.2165/11597440-000000000-00000. - DOI - PubMed
LinkOut - more resources
Full Text Sources

