Use of Plant Extracts in Polymeric Scaffolds in the Regeneration of Mandibular Injuries
- PMID: 38675152
- PMCID: PMC11053713
- DOI: 10.3390/pharmaceutics16040491
Use of Plant Extracts in Polymeric Scaffolds in the Regeneration of Mandibular Injuries
Abstract
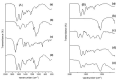
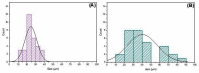
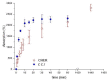
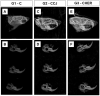
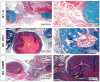
Severe loss of bone mass may require grafting, and, among the alternatives available, there are natural biomaterials that can act as scaffolds for the cell growth necessary for tissue regeneration. Collagen and elastin polymers are a good alternative due to their biomimetic properties of bone tissue, and their characteristics can be improved with the addition of polysaccharides such as chitosan and bioactive compounds such as jatoba resin and pomegranate extract due to their antigenic actions. The aim of this experimental protocol was to evaluate bone neoformation in experimentally made defects in the mandible of rats using polymeric scaffolds with plant extracts added. Thirty rats were divided into group 1, with a mandibular defect filled with a clot from the lesion and no graft implant (G1-C, n = 10); group 2, filled with collagen/chitosan/jatoba resin scaffolds (G2-CCJ, n = 10); and group 3, with collagen/nanohydroxyapatite/elastin/pomegranate extract scaffolds (G3-CHER, n = 10). Six weeks after surgery, the animals were euthanized and samples from the surgical areas were submitted to macroscopic, radiological, histological, and morphometric analysis of the mandibular lesion repair process. The results showed no inflammatory infiltrates in the surgical area, indicating good acceptance of the scaffolds in the microenvironment of the host area. In the control group (G1), there was a predominance of reactive connective tissue, while in the grafted groups (G2 and G3), there was bone formation from the margins of the lesion, but it was still insufficient for total bone repair of the defect within the experimental period standardized in this study. The histomorphometric analysis showed that the mean percentage of bone volume formed in the surgical area of groups G1, G2, and G3 was 17.17 ± 2.68, 27.45 ± 1.65, and 34.07 ± 0.64 (mean ± standard deviation), respectively. It can be concluded that these scaffolds with plant extracts added can be a viable alternative for bone repair, as they are easily manipulated, have a low production cost, and stimulate the formation of new bone by osteoconduction.
Keywords: bone regeneration; bone repair; collagen; elastin; hydroxyapatite; jatoba; plant extracts; polymers; pomegranate; scaffolds.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Collagen-chitosan-hydroxyapatite composite scaffolds for bone repair in ovariectomized rats.Sci Rep. 2023 Jan 2;13(1):28. doi: 10.1038/s41598-022-24424-x. Sci Rep. 2023. PMID: 36593236 Free PMC article.
-
Viability of Collagen Matrix Grafts Associated with Nanohydroxyapatite and Elastin in Bone Repair in the Experimental Condition of Ovariectomy.Int J Mol Sci. 2023 Oct 29;24(21):15727. doi: 10.3390/ijms242115727. Int J Mol Sci. 2023. PMID: 37958710 Free PMC article.
-
Effects of the combination of low-level laser therapy and anionic polymer membranes on bone repair.Lasers Med Sci. 2020 Jun;35(4):813-821. doi: 10.1007/s10103-019-02864-8. Epub 2019 Aug 29. Lasers Med Sci. 2020. PMID: 31463820
-
Biomaterials for periodontal regeneration: a review of ceramics and polymers.Biomatter. 2012 Oct-Dec;2(4):271-7. doi: 10.4161/biom.22948. Biomatter. 2012. PMID: 23507891 Free PMC article. Review.
-
The role of natural polymers in bone tissue engineering.J Control Release. 2021 Oct 10;338:571-582. doi: 10.1016/j.jconrel.2021.08.055. Epub 2021 Sep 2. J Control Release. 2021. PMID: 34481026 Review.
Cited by
-
Bioactivity and biomedical applications of pomegranate peel extract: a comprehensive review.Front Pharmacol. 2025 Mar 26;16:1569141. doi: 10.3389/fphar.2025.1569141. eCollection 2025. Front Pharmacol. 2025. PMID: 40206073 Free PMC article. Review.
References
-
- Al-Sabahi M.E., Jamali O.M., Shindy M.I., Moussa B.G., Amin A.A.W., Zedan M.H. Aesthetic Reconstruction of Onco-surgical Mandibular Defects Using Free Fibular Flap with and without CAD/CAM Customized Osteotomy Guide: A Randomized Controlled Clinical Trial. BMC Cancer. 2022;22:1252. doi: 10.1186/s12885-022-10322-y. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

