Insights into the Mechanism of Enhanced Dissolution in Solid Crystalline Formulations
- PMID: 38675170
- PMCID: PMC11054551
- DOI: 10.3390/pharmaceutics16040510
Insights into the Mechanism of Enhanced Dissolution in Solid Crystalline Formulations
Abstract
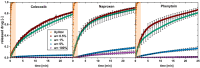
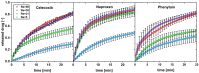
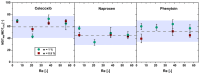
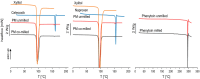
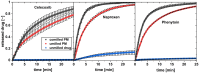
Solid dispersions are a promising approach to enhance the dissolution of poorly water-soluble drugs. Solid crystalline formulations show a fast drug dissolution and a high thermodynamic stability. To understand the mechanisms leading to the faster dissolution of solid crystalline formulations, physical mixtures of the poorly soluble drugs celecoxib, naproxen and phenytoin were investigated in the flow through cell (apparatus 4). The effect of drug load, hydrodynamics in the flow through cell and particle size reduction in co-milled physical mixtures were studied. A carrier- and drug-enabled dissolution could be distinguished. Below a certain drug load, the limit of drug load, carrier-enabled dissolution occurred, and above this value, the drug defined the dissolution rate. For a carrier-enabled behavior, the dissolution kinetics can be divided into a first fast phase, a second slow phase and a transition phase in between. This study contributes to the understanding of the dissolution mechanism in solid crystalline formulations and is thereby valuable for the process and formulation development.
Keywords: dissolution; dissolution enhancement; dissolution mechanism; flow through cell; formulation strategy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Lipinski C. Poor aqueous solubility—An industry wide problem in drug discovery. Am. Pharm. Rev. 2002;5:82–85.
LinkOut - more resources
Full Text Sources

