Polymeric Amorphous Solid Dispersions of Dasatinib: Formulation and Ecotoxicological Assessment
- PMID: 38675212
- PMCID: PMC11053848
- DOI: 10.3390/pharmaceutics16040551
Polymeric Amorphous Solid Dispersions of Dasatinib: Formulation and Ecotoxicological Assessment
Abstract
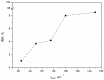
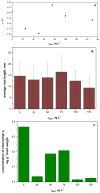
Dasatinib (DAS), a potent anticancer drug, has been subjected to formulation enhancements due to challenges such as significant first-pass metabolism, poor absorption, and limited oral bioavailability. To improve its release profile, DAS was embedded in a matrix of the hydrophilic polymer polyvinylpyrrolidone (PVP). Drug amorphization was induced in a planetary ball mill by solvent-free co-grinding, facilitating mechanochemical activation. This process resulted in the formation of amorphous solid dispersions (ASDs). The ASD capsules exhibited a notable enhancement in the release rate of DAS compared to capsules containing the initial drug. Given that anticancer drugs often undergo limited metabolism in the body with unchanged excretion, the ecotoxicological effect of the native form of DAS was investigated as well, considering its potential accumulation in the environment. The highest ecotoxicological effect was observed on the bacteria Vibrio fischeri, while other test organisms (bacteria Pseudomonas putida, microalgae Chlorella sp., and duckweed Lemna minor) exhibited negligible effects. The enhanced drug release not only contributes to improved oral absorption but also has the potential to reduce the proportion of DAS that enters the environment through human excretion. This comprehensive approach highlights the significance of integrating advances in drug development while considering its environmental implications.
Keywords: amorphous solid dispersion; dasatinib; drug release; ecotoxicological evaluation; mechanochemical activation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Insights into the Dissolution Mechanism of Ritonavir-Copovidone Amorphous Solid Dispersions: Importance of Congruent Release for Enhanced Performance.Mol Pharm. 2019 Mar 4;16(3):1327-1339. doi: 10.1021/acs.molpharmaceut.8b01261. Epub 2019 Feb 5. Mol Pharm. 2019. PMID: 30669846
-
Ternary Solid Dispersions: A Review of the Preparation, Characterization, Mechanism of Drug Release, and Physical Stability.Pharmaceutics. 2023 Aug 10;15(8):2116. doi: 10.3390/pharmaceutics15082116. Pharmaceutics. 2023. PMID: 37631330 Free PMC article. Review.
-
Amorphous solid dispersion of nisoldipine by solvent evaporation technique: preparation, characterization, in vitro, in vivo evaluation, and scale up feasibility study.Drug Deliv Transl Res. 2020 Aug;10(4):903-918. doi: 10.1007/s13346-020-00775-8. Drug Deliv Transl Res. 2020. PMID: 32378174
-
Formulation Characterization and Pharmacokinetic Evaluation of Amorphous Solid Dispersions of Dasatinib.Pharmaceutics. 2022 Nov 13;14(11):2450. doi: 10.3390/pharmaceutics14112450. Pharmaceutics. 2022. PMID: 36432641 Free PMC article.
-
Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs.J Pharm Sci. 2016 Sep;105(9):2527-2544. doi: 10.1016/j.xphs.2015.10.008. Epub 2016 Jan 23. J Pharm Sci. 2016. PMID: 26886314 Review.
References
-
- Shah N.P., Rousselot P., Schiffer C., Rea D., Cortes J.E., Milone J., Mohamed H., Healey D., Kantarjian H., Hochhaus A., et al. Dasatinib in Imatinib-Resistant or -Intolerant Chronic-Phase, Chronic Myeloid Leukemia Patients: 7-Year Follow-up of Study CA180-034. Am. J. Hematol. 2016;91:869–874. doi: 10.1002/ajh.24423. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

