Testing S. sonnei GMMA with and without Aluminium Salt-Based Adjuvants in Animal Models
- PMID: 38675229
- PMCID: PMC11054012
- DOI: 10.3390/pharmaceutics16040568
Testing S. sonnei GMMA with and without Aluminium Salt-Based Adjuvants in Animal Models
Abstract
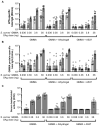
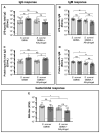
Shigellosis is one of the leading causes of diarrheal disease in low- and middle-income countries, particularly in young children, and is more often associated with antimicrobial resistance. Therefore, a preventive vaccine against shigellosis is an urgent medical need. We have proposed Generalised Modules for Membrane Antigens (GMMA) as an innovative delivery system for Shigella sonnei O-antigen, and an Alhydrogel formulation (1790GAHB) has been extensively tested in preclinical and clinical studies. Alhydrogel has been used as an adsorbent agent with the main purpose of reducing potential GMMA systemic reactogenicity. However, the immunogenicity and systemic reactogenicity of this GMMA-based vaccine formulated with or without Alhydrogel have never been compared. In this work, we investigated the potential adjuvant effect of aluminium salt-based adjuvants (Alhydrogel and AS37) on S. sonnei GMMA immunogenicity in mice and rabbits, and we found that S. sonnei GMMA alone resulted to be strongly immunogenic. The addition of neither Alhydrogel nor AS37 improved the magnitude or the functionality of vaccine-elicited antibodies. Interestingly, rabbits injected with either S. sonnei GMMA adsorbed on Alhydrogel or S. sonnei GMMA alone showed a limited and transient body temperature increase, returning to baseline values within 24 h after each vaccination. Overall, immunisation with unadsorbed GMMA did not raise any concern for animal health. We believe that these data support the clinical testing of GMMA formulated without Alhydrogel, which would allow for further simplification of GMMA-based vaccine manufacturing.
Keywords: GMMA-based vaccine; OMV; adjuvant; immune response; unadsorbed GMMA.
Conflict of interest statement
Valentina Caradonna is a student at the University of Siena and participates in a postgraduate studentship program at GSK. All the other authors are employed by the GSK group of companies.
Figures



Similar articles
-
Protein-specific immune response elicited by the Shigella sonnei 1790GAHB GMMA-based candidate vaccine in adults with varying exposure to Shigella.mSphere. 2025 May 27;10(5):e0105724. doi: 10.1128/msphere.01057-24. Epub 2025 Apr 16. mSphere. 2025. PMID: 40237462 Free PMC article.
-
Development of FAcE (Formulated Alhydrogel competitive ELISA) method for direct quantification of OAg present in Shigella sonnei GMMA-based vaccine and its optimization using Design of Experiments approach.J Immunol Methods. 2019 Aug;471:11-17. doi: 10.1016/j.jim.2019.04.012. Epub 2019 Apr 27. J Immunol Methods. 2019. PMID: 31039338
-
Production of a Shigella sonnei Vaccine Based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB.PLoS One. 2015 Aug 6;10(8):e0134478. doi: 10.1371/journal.pone.0134478. eCollection 2015. PLoS One. 2015. PMID: 26248044 Free PMC article.
-
Towards a Four-Component GMMA-Based Vaccine against Shigella.Vaccines (Basel). 2022 Feb 18;10(2):328. doi: 10.3390/vaccines10020328. Vaccines (Basel). 2022. PMID: 35214786 Free PMC article. Review.
-
GMMA-Based Vaccines: The Known and The Unknown.Front Immunol. 2021 Aug 3;12:715393. doi: 10.3389/fimmu.2021.715393. eCollection 2021. Front Immunol. 2021. PMID: 34413858 Free PMC article. Review.
Cited by
-
Exploration of a GMMA-Based Bivalent Vaccine Against Klebsiella pneumoniae.Vaccines (Basel). 2025 Feb 24;13(3):226. doi: 10.3390/vaccines13030226. Vaccines (Basel). 2025. PMID: 40266066 Free PMC article.
References
-
- WHO . Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. WHO; Geneva, Switzerland: 2017.
-
- Troeger C., Blacker B.F., Khalil I.A., Rao P.C., Cao S., Zimsen S.R.M., Albertson S.B., Stanaway J.D., Deshpande A., Abebe Z., et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018;18:1211–1228. doi: 10.1016/S1473-3099(18)30362-1. - DOI - PMC - PubMed
-
- Kotloff K.L., Nasrin D., Blackwelder W.C., Wu Y., Farag T., Panchalingham S., Sow S.O., Sur D., Zaidi A.K.M., Faruque A.S.G., et al. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: A 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS) Lancet Glob. Health. 2019;7:e568–e584. doi: 10.1016/S2214-109X(19)30076-2. - DOI - PMC - PubMed
-
- Khalil I.A., Troeger C., Blacker B.F., Rao P.C., Brown A., Atherly D.E., Brewer T.G., Engmann C.M., Houpt E.R., Kang G., et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018;18:1229–1240. - PMC - PubMed
-
- Cohen D., Meron-Sudai S., Bialik A., Asato V., Goren S., Ariel-Cohen O., Reizis A., Hochberg A., Ashkenazi S. Serum IgG antibodies to Shigella lipopolysaccharide antigens—A correlate of protection against shigellosis. Hum. Vaccines Immunother. 2019;15:1401–1408. doi: 10.1080/21645515.2019.1606971. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

