Fabrication of a Three-Dimensional Microfluidic System from Poly(methyl methacrylate) (PMMA) Using an Intermiscibility Vacuum Bonding Technique
- PMID: 38675265
- PMCID: PMC11052095
- DOI: 10.3390/mi15040454
Fabrication of a Three-Dimensional Microfluidic System from Poly(methyl methacrylate) (PMMA) Using an Intermiscibility Vacuum Bonding Technique
Abstract
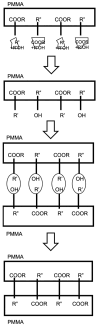
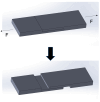
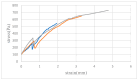
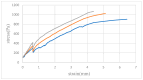
In this study, the fabrication of microfluidic chips through the bonding of poly (methyl methacrylate) (PMMA) boards featuring designed patterns to create a three-dimensional sandwich structure with embedded microchannels was explored. A key focus was optimization of the interface quality of bonded PMMA pairs by adjusting the solvent, such as such as acetone, alcohol, and their mixture. Annealing was conducted below 50 °C to leverage the advantages of low-temperature bonding. Because of the differences in the chemical reactivity of PMMA toward acetone, alcohol, and their combinations, the resulting defect densities at the bonding interfaces differed significantly under low-temperature annealing conditions. To achieve the optimal sealing integrity, bonding pressures of 30 N, 40 N, and 50 N were evaluated. The interface was analyzed through microstructural examination via optical microscopy and stress measurements were determined using digital photoelasticity, while the bonding strength was assessed through tensile testing.
Keywords: PMMA bonding; digital photoelasticity analysis; intermiscibility bonding technique; microfluidic chip fabrication; solvent miscibility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Thermal assisted ultrasonic bonding method for poly(methyl methacrylate) (PMMA) microfluidic devices.Talanta. 2010 Jun 15;81(4-5):1331-8. doi: 10.1016/j.talanta.2010.02.031. Epub 2010 Feb 13. Talanta. 2010. PMID: 20441903
-
Microfabrication of Nonplanar Polymeric Microfluidics.Micromachines (Basel). 2018 Sep 25;9(10):491. doi: 10.3390/mi9100491. Micromachines (Basel). 2018. PMID: 30424424 Free PMC article.
-
Plasticizer-assisted bonding of poly(methyl methacrylate) microfluidic chips at low temperature.J Chromatogr A. 2010 Jan 1;1217(1):160-6. doi: 10.1016/j.chroma.2009.11.018. Epub 2009 Nov 10. J Chromatogr A. 2010. PMID: 19945714
-
Fabrication of a microfluidic system for capillary electrophoresis using a two-stage embossing technique and solvent welding on poly(methyl methacrylate) with water as a sacrificial layer.Anal Chem. 2008 Apr 1;80(7):2311-8. doi: 10.1021/ac7021647. Epub 2008 Feb 28. Anal Chem. 2008. PMID: 18303914
-
Rapid Fabrication of Poly(methyl methacrylate) Devices for Lab-on-a-Chip Applications Using Acetic Acid and UV Treatment.ACS Omega. 2020 Jul 8;5(28):17396-17404. doi: 10.1021/acsomega.0c01770. eCollection 2020 Jul 21. ACS Omega. 2020. PMID: 32715224 Free PMC article.
Cited by
-
Zweifach-Fung Microfluidic Device for Efficient Microparticle Separation: Cost-Effective Fabrication Using CO2 Laser-Ablated PMMA.Micromachines (Basel). 2024 Jul 22;15(7):932. doi: 10.3390/mi15070932. Micromachines (Basel). 2024. PMID: 39064443 Free PMC article.
-
Recent Advances in Polymer Science and Fabrication Processes for Enhanced Microfluidic Applications: An Overview.Micromachines (Basel). 2024 Sep 6;15(9):1137. doi: 10.3390/mi15091137. Micromachines (Basel). 2024. PMID: 39337797 Free PMC article. Review.
-
Nonthermal Effect of Microwave Processing Enhances Interface Reactivity and Microchannel Integrity: Low-Temperature Rapid Bonding of PMMA Microfluidic Devices.ACS Omega. 2024 Dec 18;10(8):7662-7671. doi: 10.1021/acsomega.4c07013. eCollection 2025 Mar 4. ACS Omega. 2024. PMID: 40060821 Free PMC article.
References
-
- Diehl R.O., Lima R.A.M.M., Catarino S.O. Study of the development of PDMS microchannels for incorporation in masks. In i9MASKS Workshop: Extended Abstracts. 2020. [(accessed on 28 February 2024)]. p. 44. Available online: https://ebooks.uminho.pt/index.php/uminho/catalog/download/39/69/1369?in....
-
- Volpe A., Krishnan U., Chiriacò M.S., Primiceri E., Ancona A., Ferrara F. A smart procedure for the femtosecond laser-based fabrication of a polymeric lab-on-a-chip for capturing tumor cell. Engineering. 2021;7:1434–1440. doi: 10.1016/j.eng.2020.10.012. - DOI
-
- Sakamoto H., Hatsuda R., Miyamura K., Sugiyama S. Plasma separation PMMA device driven by capillary force controlling surface wettability. Micro Nano Lett. 2012;7:64–67. doi: 10.1049/mnl.2011.0627. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

