Coaxial 3D Bioprinting Process Research and Performance Tests on Vascular Scaffolds
- PMID: 38675274
- PMCID: PMC11051886
- DOI: 10.3390/mi15040463
Coaxial 3D Bioprinting Process Research and Performance Tests on Vascular Scaffolds
Abstract
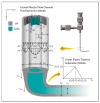
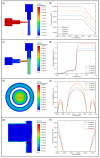
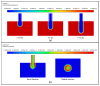
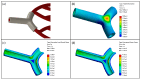
Three-dimensionally printed vascularized tissue, which is suitable for treating human cardiovascular diseases, should possess excellent biocompatibility, mechanical performance, and the structure of complex vascular networks. In this paper, we propose a method for fabricating vascularized tissue based on coaxial 3D bioprinting technology combined with the mold method. Sodium alginate (SA) solution was chosen as the bioink material, while the cross-linking agent was a calcium chloride (CaCl2) solution. To obtain the optimal parameters for the fabrication of vascular scaffolds, we first formulated theoretical models of a coaxial jet and a vascular network. Subsequently, we conducted a simulation analysis to obtain preliminary process parameters. Based on the aforementioned research, experiments of vascular scaffold fabrication based on the coaxial jet model and experiments of vascular network fabrication were carried out. Finally, we optimized various parameters, such as the flow rate of internal and external solutions, bioink concentration, and cross-linking agent concentration. The performance tests showed that the fabricated vascular scaffolds had levels of satisfactory degradability, water absorption, and mechanical properties that meet the requirements for practical applications. Cellular experiments with stained samples demonstrated satisfactory proliferation of human umbilical vein endothelial cells (HUVECs) within the vascular scaffold over a seven-day period, observed under a fluorescent inverted microscope. The cells showed good biocompatibility with the vascular scaffold. The above results indicate that the fabricated vascular structure initially meet the requirements of vascular scaffolds.
Keywords: 3D bioprinting; biological scaffold; coaxial jet; finite element analysis; vascular network.
Conflict of interest statement
The authors declare no conflict of interest. Rougang Zhou is employee of Mstar Technologies, Inc. The paper reflects the views of the scientists, and not the company.
Figures


Similar articles
-
Directly coaxial bioprinting of 3D vascularized tissue using novel bioink based on decellularized human amniotic membrane.Int J Biol Macromol. 2023 Dec 31;253(Pt 4):127041. doi: 10.1016/j.ijbiomac.2023.127041. Epub 2023 Sep 22. Int J Biol Macromol. 2023. PMID: 37742904
-
Coaxial 3D bioprinting of tri-polymer scaffolds to improve the osteogenic and vasculogenic potential of cells in co-culture models.J Biomed Mater Res A. 2022 May;110(5):1077-1089. doi: 10.1002/jbm.a.37354. Epub 2022 Jan 13. J Biomed Mater Res A. 2022. PMID: 35025130
-
Three-Dimensional Bioprinting of Perfusable Hierarchical Microchannels with Alginate and Silk Fibroin Double Cross-linked Network.3D Print Addit Manuf. 2020 Apr 1;7(2):78-84. doi: 10.1089/3dp.2019.0115. Epub 2020 Apr 16. 3D Print Addit Manuf. 2020. PMID: 36654759 Free PMC article.
-
ECM Based Bioink for Tissue Mimetic 3D Bioprinting.Adv Exp Med Biol. 2018;1064:335-353. doi: 10.1007/978-981-13-0445-3_20. Adv Exp Med Biol. 2018. PMID: 30471042 Review.
-
Bioprinting for vascular and vascularized tissue biofabrication.Acta Biomater. 2017 Mar 15;51:1-20. doi: 10.1016/j.actbio.2017.01.035. Epub 2017 Jan 11. Acta Biomater. 2017. PMID: 28087487 Review.
Cited by
-
Formation of Stable Vascular Networks by 3D Coaxial Printing and Schiff-Based Reaction.Gels. 2024 May 25;10(6):366. doi: 10.3390/gels10060366. Gels. 2024. PMID: 38920913 Free PMC article.
-
Leveraging printability and biocompatibility in materials for printing implantable vessel scaffolds.Mater Today Bio. 2024 Nov 23;29:101366. doi: 10.1016/j.mtbio.2024.101366. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39698000 Free PMC article. Review.
-
Hydrogel-Based Bioinks for Coaxial and Triaxial Bioprinting: A Review of Material Properties, Printing Techniques, and Applications.Polymers (Basel). 2025 Mar 28;17(7):917. doi: 10.3390/polym17070917. Polymers (Basel). 2025. PMID: 40219306 Free PMC article. Review.
-
The Promise and Challenges of Bioprinting in Tissue Engineering.Micromachines (Basel). 2024 Dec 23;15(12):1529. doi: 10.3390/mi15121529. Micromachines (Basel). 2024. PMID: 39770282 Free PMC article.
References
-
- Li Z., Li X., Xu T., Zhang L. Acellular Small-Diameter Tissue-Engineered Vascular Grafts. Appl. Sci. 2019;9:2864. doi: 10.3390/app9142864. - DOI
-
- O’Doherty M.G., Cairns K., O’Neill V., Lamrock F., Jørgensen T., Brenner H., Schöttker B., Wilsgaard T., Siganos G., Kuulasmaa K., et al. Effect of major lifestyle risk factors, independent and jointly, on life expectancy with and without cardiovascular disease: Results from the Consortium on Health and Ageing Network of Cohorts in Europe and the United States (CHANCES) Eur. J. Epidemiol. 2016;31:455–468. doi: 10.1007/s10654-015-0112-8. - DOI - PMC - PubMed
Grants and funding
- GZKF-202204/Open Foundation of the State Key Laboratory of Fluid Power and Mechatronic Systems
- GK229909299001-403/Basic scientific research project of provincial universities of Hangzhou Dianzi University
- Y22E055902/Zhejiang Provincial Natural Science Foundation of China
- 3DL202105/Open Foundation of the State Key Laboratory of Fluid Power and Mechatronic Systems, Jiangsu Key Laboratory of 3D Printing Equipment and Manufacturing
LinkOut - more resources
Full Text Sources

