The Xanthine Derivative KMUP-1 Inhibits Hypoxia-Induced TRPC1 Expression and Store-Operated Ca2+ Entry in Pulmonary Arterial Smooth Muscle Cells
- PMID: 38675401
- PMCID: PMC11053947
- DOI: 10.3390/ph17040440
The Xanthine Derivative KMUP-1 Inhibits Hypoxia-Induced TRPC1 Expression and Store-Operated Ca2+ Entry in Pulmonary Arterial Smooth Muscle Cells
Abstract
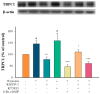
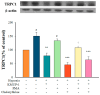
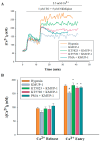
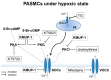
Exposure to hypoxia results in the development of pulmonary arterial hypertension (PAH). An increase in the intracellular Ca2+ concentration ([Ca2+]i) in pulmonary artery smooth muscle cells (PASMCs) is a major trigger for pulmonary vasoconstriction and proliferation. This study investigated the mechanism by which KMUP-1, a xanthine derivative with phosphodiesterase inhibitory activity, inhibits hypoxia-induced canonical transient receptor potential channel 1 (TRPC1) protein overexpression and regulates [Ca2+]i through store-operated calcium channels (SOCs). Ex vivo PASMCs were cultured from Sprague-Dawley rats in a modular incubator chamber under 1% O2/5% CO2 for 24 h to elucidate TRPC1 overexpression and observe the Ca2+ release and entry. KMUP-1 (1 μM) inhibited hypoxia-induced TRPC family protein encoded for SOC overexpression, particularly TRPC1. KMUP-1 inhibition of TRPC1 protein was restored by the protein kinase G (PKG) inhibitor KT5823 (1 μM) and the protein kinase A (PKA) inhibitor KT5720 (1 μM). KMUP-1 attenuated protein kinase C (PKC) activator phorbol 12-myristate 13-acetate (PMA, 1 μM)-upregulated TRPC1. We suggest that the effects of KMUP-1 on TRPC1 might involve activating the cyclic guanosine monophosphate (cGMP)/PKG and cyclic adenosine monophosphate (cAMP)/PKA pathways and inhibiting the PKC pathway. We also used Fura 2-acetoxymethyl ester (Fura 2-AM, 5 μM) to measure the stored calcium release from the sarcoplasmic reticulum (SR) and calcium entry through SOCs in hypoxic PASMCs under treatment with thapsigargin (1 μM) and nifedipine (5 μM). In hypoxic conditions, store-operated calcium entry (SOCE) activity was enhanced in PASMCs, and KMUP-1 diminished this activity. In conclusion, KMUP-1 inhibited the expression of TRPC1 protein and the activity of SOC-mediated Ca2+ entry upon SR Ca2+ depletion in hypoxic PASMCs.
Keywords: KMUP-1; canonical transient receptor potential channel 1; hypoxia; protein kinases; pulmonary artery smooth muscle cells; store-operated calcium entry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Sildenafil inhibits hypoxia-induced transient receptor potential canonical protein expression in pulmonary arterial smooth muscle via cGMP-PKG-PPARγ axis.Am J Respir Cell Mol Biol. 2013 Aug;49(2):231-40. doi: 10.1165/rcmb.2012-0185OC. Am J Respir Cell Mol Biol. 2013. PMID: 23526219 Free PMC article.
-
The Xanthine Derivative KMUP-1 Attenuates Serotonin-Induced Vasoconstriction and K⁺-Channel Inhibitory Activity via the PKC Pathway in Pulmonary Arteries.Int J Biol Sci. 2015 Apr 25;11(6):633-42. doi: 10.7150/ijbs.11127. eCollection 2015. Int J Biol Sci. 2015. PMID: 25999786 Free PMC article.
-
Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension.Circ Res. 2004 Sep 3;95(5):496-505. doi: 10.1161/01.RES.0000138952.16382.ad. Epub 2004 Jul 15. Circ Res. 2004. PMID: 15256480
-
Insights into Activation Mechanisms of Store-Operated TRPC1 Channels in Vascular Smooth Muscle.Cells. 2020 Jan 10;9(1):179. doi: 10.3390/cells9010179. Cells. 2020. PMID: 31936855 Free PMC article. Review.
-
STIM-TRP Pathways and Microdomain Organization: Contribution of TRPC1 in Store-Operated Ca2+ Entry: Impact on Ca2+ Signaling and Cell Function.Adv Exp Med Biol. 2017;993:159-188. doi: 10.1007/978-3-319-57732-6_9. Adv Exp Med Biol. 2017. PMID: 28900914 Review.
References
-
- Humbert M., Guignabert C., Bonnet S., Dorfmüller P., Klinger J.R., Nicolls M.R., Olschewski A.J., Pullamsetti S.S., Schermuly R.T., Stenmark K.R., et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019;53:1801887. doi: 10.1183/13993003.01887-2018. - DOI - PMC - PubMed
-
- Vonk Noordegraaf A., Chin K.M., Haddad F., Hassoun P.M., Hemnes A.R., Hopkins S.R., Kawut S.M., Langleben D., Lumens J., Naeije R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur. Respir. J. 2019;53:1801900. doi: 10.1183/13993003.01900-2018. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

