Effects of Ganjianglingzhu Decoction on Lean Non-Alcoholic Fatty Liver Disease in Mice Based on Untargeted Metabolomics
- PMID: 38675462
- PMCID: PMC11053674
- DOI: 10.3390/ph17040502
Effects of Ganjianglingzhu Decoction on Lean Non-Alcoholic Fatty Liver Disease in Mice Based on Untargeted Metabolomics
Abstract
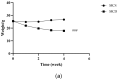
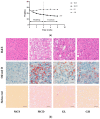
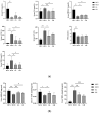
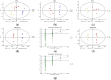
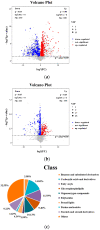
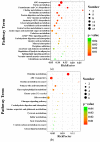
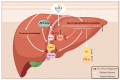
Non-alcoholic fatty liver disease (NAFLD) is usually associated with obesity. However, it is crucial to recognize that NAFLD can also occur in lean individuals, which is frequently overlooked. Without an approved pharmacological therapy for lean NAFLD, we aimed to investigate whether the Ganjianglingzhu (GJLZ) decoction, a representative traditional Chinese medicine (TCM), protects against lean NAFLD and explore the potential mechanism underlying these protective effects. The mouse model of lean NAFLD was established with a methionine-choline-deficient (MCD) diet in male C57BL/6 mice to be compared with the control group fed the methionine-choline-sufficient (MCS) diet. After four weeks, physiological saline, a low dose of GJLZ decoction (GL), or a high dose of GJLZ decoction (GH) was administered daily by gavage to the MCD group; the MCS group was given physiological saline by gavage. Untargeted metabolomics techniques were used to explore further the potential mechanism of the effects of GJLZ on lean NAFLD. Different doses of GJLZ decoction were able to ameliorate steatosis, inflammation, fibrosis, and oxidative stress in the liver; GL performed a better effect on lean NAFLD. In addition, 78 candidate differential metabolites were screened and identified. Combined with metabolite pathway enrichment analysis, GL was capable of regulating the glucose and lipid metabolite pathway in lean NAFLD and regulating the glycerophospholipid metabolism by altering the levels of sn-3-O-(geranylgeranyl)glycerol 1-phosphate and lysoPC(P-18:0/0:0). GJLZ may protect against the development of lean NAFLD by regulating glucose and lipid metabolism, inhibiting the levels of sn-3-O-(geranylgeranyl)glycerol 1-phosphate and lysoPC(P-18:0/0:0) in glycerophospholipid metabolism.
Keywords: Ganjianglingzhu decoction; glycerophospholipid metabolism; lean NAFLD.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Gan-Jiang-Ling-Zhu decoction alleviates hepatic steatosis in rats by the miR-138-5p/CPT1B axis.Biomed Pharmacother. 2020 Jul;127:110127. doi: 10.1016/j.biopha.2020.110127. Epub 2020 Apr 20. Biomed Pharmacother. 2020. PMID: 32325349
-
Gan-Jiang-Ling-Zhu decoction improves steatohepatitis induced by choline-deficient-high-fat-diet through the METTL14/N6-methyladenosine-mediated Ugt2a3 expression.J Ethnopharmacol. 2025 Jan 13;339:119153. doi: 10.1016/j.jep.2024.119153. Epub 2024 Nov 22. J Ethnopharmacol. 2025. PMID: 39580132
-
Xie Zhuo Tiao Zhi formula modulates intestinal microbiota and liver purine metabolism to suppress hepatic steatosis and pyroptosis in NAFLD therapy.Phytomedicine. 2023 Dec;121:155111. doi: 10.1016/j.phymed.2023.155111. Epub 2023 Sep 23. Phytomedicine. 2023. PMID: 37804819
-
New aspects characterizing non-obese NAFLD by the analysis of the intestinal flora and metabolites using a mouse model.mSystems. 2024 Mar 19;9(3):e0102723. doi: 10.1128/msystems.01027-23. Epub 2024 Feb 29. mSystems. 2024. PMID: 38421203 Free PMC article.
-
A Comparison of the Gene Expression Profiles of Non-Alcoholic Fatty Liver Disease between Animal Models of a High-Fat Diet and Methionine-Choline-Deficient Diet.Molecules. 2022 Jan 27;27(3):858. doi: 10.3390/molecules27030858. Molecules. 2022. PMID: 35164140 Free PMC article. Review.
Cited by
-
Metabolomic Hallmarks of Obesity and Metabolic Dysfunction-Associated Steatotic Liver Disease.Int J Mol Sci. 2024 Nov 28;25(23):12809. doi: 10.3390/ijms252312809. Int J Mol Sci. 2024. PMID: 39684520 Free PMC article. Review.
-
Gan-jiang-ling-zhu decoction improves steatohepatitis by regulating gut microbiota-mediated 12-tridecenoic acid inhibition.Front Pharmacol. 2024 Aug 23;15:1444561. doi: 10.3389/fphar.2024.1444561. eCollection 2024. Front Pharmacol. 2024. PMID: 39246653 Free PMC article.
References
-
- Riazi K., Azhari H., Charette J.H., Underwood F.E., King J.A., Afshar E.E., Swain M.G., Congly S.E., Kaplan G.G., Shaheen A.-A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. - DOI - PubMed
-
- Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2017;67:328–357. doi: 10.1002/hep.29367. - DOI - PubMed
Grants and funding
- ZY (2021-2023)-0211/Three-year action plan for Shanghai
- 81973730/National Natural Science Foundation of China
- 22010504300/Local Colleges Faculty Constitution of Shanghai MSTC 2022
- 2021 Science and Technology 02-37/Shanghai Collaborative Innovation Center for Chronic Disease Prevention and Health Services
- 2022QN014/Shanghai Health Commission for Traditional Chinese Medicine Research
- 2022M722164/China Postdoctoral Science Foundation, No.72 General Fund, 2022
- Yang Fan Project, 23YF1447700/Shanghai 2023 "Science and Technology Innovation Action Plan" Qi Ming Xing Cultivation
- GJ202303/Subject Connotation Construction Project in School of Public Health, Shanghai University of Traditional Chinese Medicine, 2023
LinkOut - more resources
Full Text Sources

