Vesicular Drug Delivery Systems: Promising Approaches in Ocular Drug Delivery
- PMID: 38675470
- PMCID: PMC11054584
- DOI: 10.3390/ph17040511
Vesicular Drug Delivery Systems: Promising Approaches in Ocular Drug Delivery
Abstract
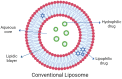
Ocular drug delivery poses unique challenges due to the complex anatomical and physiological barriers of the eye. Conventional dosage forms often fail to achieve optimal therapeutic outcomes due to poor bioavailability, short retention time, and off-target effects. In recent years, vesicular drug delivery systems have emerged as promising solutions to address these challenges. Vesicular systems, such as liposome, niosome, ethosome, transfersome, and others (bilosome, transethosome, cubosome, proniosome, chitosome, terpesome, phytosome, discome, and spanlastics), offer several advantages for ocular drug delivery. These include improved drug bioavailability, prolonged retention time on the ocular surface, reduced systemic side effects, and protection of drugs from enzymatic degradation and dilution by tears. Moreover, vesicular formulations can be engineered for targeted delivery to specific ocular tissues or cells, enhancing therapeutic efficacy while minimizing off-target effects. They also enable the encapsulation of a wide range of drug molecules, including hydrophilic, hydrophobic, and macromolecular drugs, and the possibility of combination therapy by facilitating the co-delivery of multiple drugs. This review examines vesicular drug delivery systems, their advantages over conventional drug delivery systems, production techniques, and their applications in management of ocular diseases.
Keywords: liposomes; ocular drug delivery; sustained release; targeted delivery; vesicular systems.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Approaches to Address PK-PD Challenges of Conventional Liposome Formulation with Special Reference to Cancer, Alzheimer's, Diabetes, and Glaucoma: An Update on Modified Liposomal Drug Delivery System.Curr Drug Metab. 2022;23(9):678-692. doi: 10.2174/1389200223666220609141459. Curr Drug Metab. 2022. PMID: 35692131 Review.
-
Bilosomes as a novel ocular drug delivery system: Assessing the material attributes, process parameters, and quality attributes.Exp Eye Res. 2025 Jun;255:110364. doi: 10.1016/j.exer.2025.110364. Epub 2025 Mar 27. Exp Eye Res. 2025. PMID: 40157630 Review.
-
Lipid Nanoparticles as a Promising Drug Delivery Carrier for Topical Ocular Therapy-An Overview on Recent Advances.Pharmaceutics. 2022 Feb 27;14(3):533. doi: 10.3390/pharmaceutics14030533. Pharmaceutics. 2022. PMID: 35335909 Free PMC article. Review.
-
Recent advances in ocular drug delivery.Drug Dev Ind Pharm. 2013 Nov;39(11):1599-617. doi: 10.3109/03639045.2012.736515. Epub 2012 Nov 16. Drug Dev Ind Pharm. 2013. PMID: 23153114 Review.
-
Spanlastics: a novel elastic drug delivery system with potential applications via multifarious routes of administration.J Drug Target. 2023 Dec;31(10):999-1012. doi: 10.1080/1061186X.2023.2274805. Epub 2023 Nov 29. J Drug Target. 2023. PMID: 37926975 Review.
Cited by
-
Curcumin in Ophthalmology: Mechanisms, Challenges, and Emerging Opportunities.Molecules. 2025 Jan 21;30(3):457. doi: 10.3390/molecules30030457. Molecules. 2025. PMID: 39942561 Free PMC article. Review.
-
Harnessing nanofibers for targeted delivery of phytoconstituents in age-related macular degeneration.Drug Deliv. 2025 Dec;32(1):2489491. doi: 10.1080/10717544.2025.2489491. Epub 2025 Apr 7. Drug Deliv. 2025. PMID: 40192800 Free PMC article. Review.
References
-
- Das B., Nayak A.K., Mallick S. Lipid-Based Nanocarriers for Ocular Drug Delivery: An Updated Review. J Drug Deliv. Sci. Technol. 2022;76:103780. doi: 10.1016/j.jddst.2022.103780. - DOI
Publication types
LinkOut - more resources
Full Text Sources