Investigation on the Combined Effect of Hydroxypropyl Beta-Cyclodextrin (HPβCD) and Polysorbate in Monoclonal Antibody Formulation
- PMID: 38675488
- PMCID: PMC11054243
- DOI: 10.3390/ph17040528
Investigation on the Combined Effect of Hydroxypropyl Beta-Cyclodextrin (HPβCD) and Polysorbate in Monoclonal Antibody Formulation
Abstract
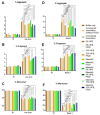
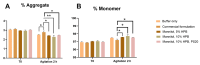
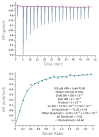
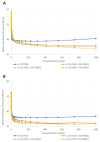
Monoclonal antibodies require careful formulation due to their inherent stability limitations. Polysorbates are commonly used to stabilize mAbs, but they are prone to degradation, which results in unwanted impurities. KLEPTOSE® HPβCD (hydroxypropyl beta-cyclodextrin) has functioned as a stable stabilizer for protein formulations in our previous research. The current study investigates the collaborative impact of combining polysorbates and HPβCD as excipients in protein formulations. The introduction of HPβCD in formulations showed it considerably reduced aggregation in two model proteins, bevacizumab and ipilimumab, following exposure to various stress conditions. The diffusion interaction parameter revealed a reduction in protein-protein interactions by HPβCD. In bevacizumab formulations, the subvisible particle counts per 0.4 mL of samples in commercial formulations vs. formulations containing both HPβCD and polysorbates subjected to distinct stressors were as follows: agitation, 87,308 particles vs. 15,350 particles; light, 25,492 particles vs. 6765 particles; and heat, 1775 particles vs. 460 particles. Isothermal titration calorimetry (ITC) measurement indicated a weak interaction between PS 80 and HPβCD, with a KD value of 74.7 ± 7.5 µM and binding sites of 5 × 10-3. Surface tension measurements illustrated that HPβCD enhanced the surface activity of polysorbates. The study suggests that combining these excipients can improve mAb stability in formulations, offering an alternative for the biopharmaceutical industry.
Keywords: KLEPTOSE® HPβCD; bevacizumab; excipient; hydroxypropyl beta-cyclodextrin; ipilimumab; monoclonal antibody; polysorbate; protein formulation; protein stability.
Conflict of interest statement
Authors Jiayi Huang, Shiqi Hong, Lucas Yuan Hao Goh, Hailong Zhang, Tao Peng, Keat Theng Chow were employed by Roquette Asia Pacific Pte Ltd., Rajeev Gokhale was employed by Roquette America Inc. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Goswami S., Wang W., Arakawa T., Ohtake S. Developments and Challenges for mAb-Based Therapeutics. Antibodies. 2013;2:452–500. doi: 10.3390/antib2030452. - DOI
LinkOut - more resources
Full Text Sources

