Electronic Effects in a Green Protocol for (Hetero)Aryl-S Coupling
- PMID: 38675533
- PMCID: PMC11051792
- DOI: 10.3390/molecules29081714
Electronic Effects in a Green Protocol for (Hetero)Aryl-S Coupling
Abstract
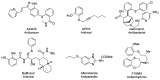
Aryl and heteroaryl iodides have been efficiently converted into the corresponding thioacetates in cyclopentyl methyl ether (CPME), a green solvent, under Cu catalysis. The chemoselectivity of the reaction is mainly controlled by electronic factors, enabling the conversion of both electron-rich and electron-deficient substrates into the corresponding thioacetates in good to excellent yields. The products can be easily deprotected to the corresponding thiolates to carry out additional synthetic transformations in situ. Surprisingly, despite CPME's relatively low dielectric constant, the reaction rate significantly increased when conducted under microwave irradiation conditions. This synthetic methodology exhibits a remarkable tolerance to functional groups, mild reaction conditions, and a wide substrate scope, utilizing a safe and inexpensive CuI pre-catalyst in the green solvent CPME. A non-aqueous workup allowing for the complete recovery of both catalyst and solvent makes this approach an environmentally sustainable protocol for C(sp2) sulfur functionalization. Additionally, the reaction shows selective cross-coupling with iodides in competition with chlorides and bromides, allowing its use in multistep syntheses. To demonstrate the potential of this methodology, it was applied to the high-yield synthesis of a photochromic dithienylethene, where a selective synthesis had not been reported before.
Keywords: Ar-S coupling; CPME; Cu catalysis; electronic effects; green chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Dubey A.V., Gharat S.B., Vijay Kumar A. Glycerol as a Recyclable Solvent for Copper-Mediated Ligand-Free C-S Cross-Coupling Reaction: Application to Synthesis of Gemmacin Precursor. ChemistrySelect. 2017;2:4852–4856. doi: 10.1002/slct.201700684. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

