A Novel Non-Psychoactive Fatty Acid from a Marine Snail, Conus inscriptus, Signals Cannabinoid Receptor 1 (CB1) to Accumulate Apoptotic C16:0 and C18:0 Ceramides in Teratocarcinoma Cell Line PA1
- PMID: 38675558
- PMCID: PMC11052367
- DOI: 10.3390/molecules29081737
A Novel Non-Psychoactive Fatty Acid from a Marine Snail, Conus inscriptus, Signals Cannabinoid Receptor 1 (CB1) to Accumulate Apoptotic C16:0 and C18:0 Ceramides in Teratocarcinoma Cell Line PA1
Abstract
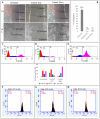
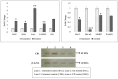
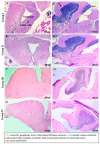
The cannabinoid-type I (CB1) receptor functions as a double-edged sword to decide cell fate: apoptosis/survival. Elevated CB1 receptor expression is shown to cause acute ceramide accumulation to meet the energy requirements of fast-growing cancers. However, the flip side of continual CB1 activation is the initiation of a second ceramide peak that leads to cell death. In this study, we used ovarian cancer cells, PA1, which expressed CB1, which increased threefold when treated with a natural compound, bis(palmitoleic acid) ester of a glycerol (C2). This novel compound is isolated from a marine snail, Conus inscriptus, using hexane and the structural details are available in the public domain PubChem database (ID: 14275348). The compound induced two acute ceramide pools to cause G0/G1 arrest and killed cells by apoptosis. The compound increased intracellular ceramides (C:16 to 7 times and C:18 to 10 times), both of which are apoptotic inducers in response to CB1 signaling and thus the compound is a potent CB1 agonist. The compound is not genotoxic because it did not induce micronuclei formation in non-cancerous Chinese hamster ovarian (CHO) cells. Since the compound induced the cannabinoid pathway, we tested if there was a psychotropic effect in zebrafish models, however, it was evident that there were no observable neurobehavioral changes in the treatment groups. With the available data, we propose that this marine compound is safe to be used in non-cancerous cells as well as zebrafish. Thus, this anticancer compound is non-toxic and triggers the CB1 pathway without causing psychotropic effects.
Keywords: FAAH1; MD simulation; apoptosis; cannabinoid receptor 1 (CB1); ceramide; ovarian cancer; zebrafish models.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures


Similar articles
-
3-Hydroxypropane-1,2-Diyl Dipalmitoleate-A Natural Compound with Dual Roles (CB1 Agonist/FAAH1 Blocker) in Inhibiting Ovarian Cancer Cell Line.Pharmaceuticals (Basel). 2021 Mar 12;14(3):255. doi: 10.3390/ph14030255. Pharmaceuticals (Basel). 2021. PMID: 33809034 Free PMC article.
-
Cannabinoid receptor activation induces apoptosis through tumor necrosis factor alpha-mediated ceramide de novo synthesis in colon cancer cells.Clin Cancer Res. 2008 Dec 1;14(23):7691-700. doi: 10.1158/1078-0432.CCR-08-0799. Clin Cancer Res. 2008. PMID: 19047095
-
Cannabinoid receptor agonists from Conus venoms alleviate pain-related behavior in rats.Pharmacol Biochem Behav. 2021 Jun;205:173182. doi: 10.1016/j.pbb.2021.173182. Epub 2021 Mar 25. Pharmacol Biochem Behav. 2021. PMID: 33774007
-
N-Palmitoyl Serinol Stimulates Ceramide Production through a CB1-Dependent Mechanism in In Vitro Model of Skin Inflammation.Int J Mol Sci. 2021 Aug 2;22(15):8302. doi: 10.3390/ijms22158302. Int J Mol Sci. 2021. PMID: 34361066 Free PMC article.
-
Cannabinoids and ceramide: two lipids acting hand-by-hand.Life Sci. 2005 Aug 19;77(14):1723-31. doi: 10.1016/j.lfs.2005.05.015. Life Sci. 2005. PMID: 15958274 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

