Dendritic Pyridine-Imine Copper Complexes as Metallo-Drugs
- PMID: 38675623
- PMCID: PMC11052306
- DOI: 10.3390/molecules29081800
Dendritic Pyridine-Imine Copper Complexes as Metallo-Drugs
Abstract
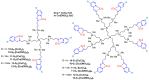
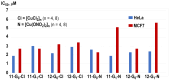
Since the discovery of cisplatin in the 1960s, the search for metallo-drugs that are more efficient than platinum complexes with negligible side effects has attracted much interest. Among the other metals that have been examined for potential applications as anticancer agents is copper. The interest in copper was recently boosted by the discovery of cuproptosis, a recently evidenced form of cell death mediated by copper. However, copper is also known to induce the proliferation of cancer cells. In view of these contradictory results, there is a need to find the most suitable copper chelators, among which Schiff-based derivatives offer a wide range of possibilities. Gathering several metal complexes in a single, larger entity may provide enhanced properties. Among the nanometric objects suitable for such purpose are dendrimers, precisely engineered hyperbranched macromolecules, which are outstanding candidates for improving therapy and diagnosis. In this review article, we present an overview of the use of a particular Schiff base, namely pyridine-imine, linked to the surface of dendrimers, suitable for complexing copper, and the use of such dendrimer complexes in biology, in particular against cancers.
Keywords: PAMAM; Schiff base; cancer; carbosilane; copper; dendrimer; metallo-drugs; phosphorus; pyridine–imine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




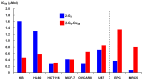


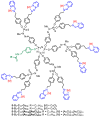
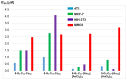


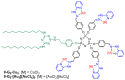



References
-
- Temesgen A., Ananda Murthy H.C., Zereffa Enyew A., Revathi R., Venkatesha Perumal R. Emerging trends in metal-based anticancer agents: Drug design to clinical trials and their mechanism of action. Chem. Select. 2023;8:e202302113. doi: 10.1002/slct.202302113. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

