The DBB Family in Populus trichocarpa: Identification, Characterization, Evolution and Expression Profiles
- PMID: 38675643
- PMCID: PMC11054233
- DOI: 10.3390/molecules29081823
The DBB Family in Populus trichocarpa: Identification, Characterization, Evolution and Expression Profiles
Abstract
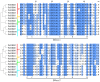
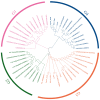
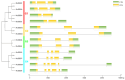
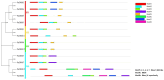
The B-box proteins (BBXs) encode a family of zinc-finger transcription factors that regulate the plant circadian rhythm and early light morphogenesis. The double B-box (DBB) family is in the class of the B-box family, which contains two conserved B-box domains and lacks a CCT (CO, CO-like and TOC1) motif. In this study, the identity, classification, structures, conserved motifs, chromosomal location, cis elements, duplication events, and expression profiles of the PtrDBB genes were analyzed in the woody model plant Populus trichocarpa. Here, 12 PtrDBB genes (PtrDBB1-PtrDBB12) were identified and classified into four distinct groups, and all of them were homogeneously spread among eight out of seventeen poplar chromosomes. The collinearity analysis of the DBB family genes from P. trichocarpa and two other species (Z. mays and A. thaliana) indicated that segmental duplication gene pairs and high-level conservation were identified. The analysis of duplication events demonstrates an insight into the evolutionary patterns of DBB genes. The previously published transcriptome data showed that PtrDBB genes represented distinct expression patterns in various tissues at different stages. In addition, it was speculated that several PtrDBBs are involved in the responsive to drought stress, light/dark, and ABA and MeJA treatments, which implied that they might function in abiotic stress and phytohormone responses. In summary, our results contribute to the further understanding of the DBB family and provide a reference for potential functional studies of PtrDBB genes in P. trichocarpa.
Keywords: DBB; Populus trichocarpa; expression patterns; phylogenetic relationships; stress response.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Zhao T., Wu T., Zhang J., Wang Z., Pei T., Yang H., Li J., Xu X. Genome-wide analyses of the genetic screening of C2H2-type zinc finger transcription factors and abiotic and biotic stress responses in tomato (Solanum iycopersicum) based on RNA-Seq data. Front. Genet. 2020;11:540. doi: 10.3389/fgene.2020.00540. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

