CB1 Receptor Negative Allosteric Modulators as a Potential Tool to Reverse Cannabinoid Toxicity
- PMID: 38675703
- PMCID: PMC11053441
- DOI: 10.3390/molecules29081881
CB1 Receptor Negative Allosteric Modulators as a Potential Tool to Reverse Cannabinoid Toxicity
Abstract
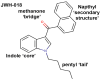
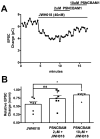
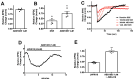
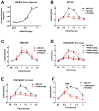
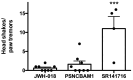
While the opioid crisis has justifiably occupied news headlines, emergency rooms are seeing many thousands of visits for another cause: cannabinoid toxicity. This is partly due to the spread of cheap and extremely potent synthetic cannabinoids that can cause serious neurological and cardiovascular complications-and deaths-every year. While an opioid overdose can be reversed by naloxone, there is no analogous treatment for cannabis toxicity. Without an antidote, doctors rely on sedatives, with their own risks, or 'waiting it out' to treat these patients. We have shown that the canonical synthetic 'designer' cannabinoids are highly potent CB1 receptor agonists and, as a result, competitive antagonists may struggle to rapidly reverse an overdose due to synthetic cannabinoids. Negative allosteric modulators (NAMs) have the potential to attenuate the effects of synthetic cannabinoids without having to directly compete for binding. We tested a group of CB1 NAMs for their ability to reverse the effects of the canonical synthetic designer cannabinoid JWH018 in vitro in a neuronal model of endogenous cannabinoid signaling and also in vivo. We tested ABD1085, RTICBM189, and PSNCBAM1 in autaptic hippocampal neurons that endogenously express a retrograde CB1-dependent circuit that inhibits neurotransmission. We found that all of these compounds blocked/reversed JWH018, though some proved more potent than others. We then tested whether these compounds could block the effects of JWH018 in vivo, using a test of nociception in mice. We found that only two of these compounds-RTICBM189 and PSNCBAM1-blocked JWH018 when applied in advance. The in vitro potency of a compound did not predict its in vivo potency. PSNCBAM1 proved to be the more potent of the compounds and also reversed the effects of JWH018 when applied afterward, a condition that more closely mimics an overdose situation. Lastly, we found that PSNCBAM1 did not elicit withdrawal after chronic JWH018 treatment. In summary, CB1 NAMs can, in principle, reverse the effects of the canonical synthetic designer cannabinoid JWH018 both in vitro and in vivo, without inducing withdrawal. These findings suggest a novel pharmacological approach to at last provide a tool to counter cannabinoid toxicity.
Keywords: JWH018; antidote; cannabinoid toxicity; overdose; synthetic cannabinoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

