A Bacterium-like Particle Vaccine Displaying Envelope Proteins of Canine Distemper Virus Can Induce Immune Responses in Mice and Dogs
- PMID: 38675892
- PMCID: PMC11055036
- DOI: 10.3390/v16040549
A Bacterium-like Particle Vaccine Displaying Envelope Proteins of Canine Distemper Virus Can Induce Immune Responses in Mice and Dogs
Abstract
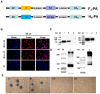
Canine distemper virus (CDV) can cause fatal infections in giant pandas. Vaccination is crucial to prevent CDV infection in giant pandas. In this study, two bacterium-like particle vaccines F3-GEM and H4-GEM displaying the trimeric F protein or tetrameric H protein of CDV were constructed based on the Gram-positive enhanced-matrix protein anchor (GEM-PA) surface display system. Electron microscopy and Western blot results revealed that the F or H protein was successfully anchored on the surface of GEM particles. Furthermore, one more bacterium-like particle vaccine F3 and H4-GEM was also designed, a mixture consisting of F3-GEM and H4-GEM at a ratio of 1:1. To evaluate the effect of the three vaccines, mice were immunized with F3-GEM, H4-GEM or F3 and H4-GEM. It was found that the level of IgG-specific antibodies and neutralizing antibodies in the F3 and H4-GEM group was higher than the other two groups. Additionally, F3 and H4-GEM also increased the secretion of Th1-related and Th2-related cytokines. Moreover, F3 and H4-GEM induce IgG and neutralizing antibodies' response in dogs. Conclusions: In summary, F3 and H4-GEM can provoke better immune responses to CDV in mice and dogs. The bacterium-like particle vaccine F3 and H4-GEM might be a potential vaccine candidate for giant pandas against CDV infection.
Keywords: CDV; F protein; H protein; bacterium-like particles; pandas; subunit vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Sheldon J.D., Cushing A.C., Wilkes R.P., Anis E., Dubovi E.J. Serologic response to canine distemper vaccination in captive linnaeus’s two-toed sloths (choloepus didactylus) after a fatal canine distemper virus outbreak. J. Zoo Wildl. Med. 2017;48:1250–1253. doi: 10.1638/1042-7260-48.4.1250. - DOI - PubMed
-
- Michelazzo M.M.Z., Oliveira T.E.S., Viana N.E., Moraes W., Cubas Z.S., Headley S.A. Immunohistochemical evidence of canine morbillivirus (canine distemper) infection in coatis (Nasua nasua) from Southern Brazil. Transbound. Emerg. Dis. 2020;67((Suppl. S2)):178–184. doi: 10.1111/tbed.13456. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

